
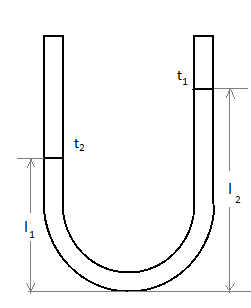
In a vertical U-tube containing a liquid, the two arms are maintained at different temperatures \[{t_1}\] and \[{t_2}\]. The liquid columns in the two arms have heights \[{l_1}\] and \[{l_2}\] respectively. The coefficient of volume expansion of liquid is equal to:
(A) \[{\dfrac{{{l_1} - {l_2}}}{{{l_2}{t_1} + {l_1}t}}_2}\]
(B) \[{\dfrac{{{l_1} - {l_2}}}{{{l_2}{t_1} - {l_1}t}}_2}\]
(C) \[{\dfrac{{{l_1} + {l_2}}}{{{l_2}{t_1} + {l_1}t}}_2}\]
(D) \[{\dfrac{{{l_1} + {l_2}}}{{{l_1}{t_1} + {l_2}t}}_2}\]
Answer
232.8k+ views
Hint: In any question involving U-tube, at first the pressure on both the arms must be balanced. In this case, after balancing the pressure on two legs apply the required conditions of volume expansion and thus obtain the coefficient of volume expansion of the liquid.
Complete step by step solution:
In a U-tube, when pressure is applied the height of liquid at the two limbs are unequal.
Let us consider, in the beginning that the pressure in both the limbs are equal:
Thus, the pressure in the limbs are balanced, so we write:
\[{p_o} + {\rho _{t_2}}g{l_2} = {p_o} + {\rho _{t_1}}g{l_1}\]
Where,
\[{p_o} = \] Atmospheric Pressure.
\[{\rho _{t_2}} = \]Density of liquid at temperature\[{t_2}\].
\[g = \] Acceleration due to gravity.
\[{l_2} = \]Length of second limb.
\[{\rho _{t_1}} = \] Density of liquid at \[{t_2}\].
\[{l_1} = \] Length of first limb.
On cancelling the common terms from sides:
\[{\rho _{t_2}}{l_2} = {\rho _{t1}}{l_1}\]
We know, due to change in temperature, the density of the liquid changes.
Now, let us consider \[{\rho _o}\] to be the initial density of the liquid.
Now, applying the formula that equates density to temperature, we know:
\[{\rho _{t_2}} = \dfrac{{{\rho _o}}}{{1 + \gamma {t_{_2}}}}\]
And, \[{\rho _{t_1}} = \dfrac{{{\rho _o}}}{{1 + \gamma {t_{_1}}}}\]
Where, \[\gamma = \] Coefficient of volume expansion.
When there is a change in volume of a liquid on application of heat (rise or reduction in temperature), the ratio of increase in volume with respect to per degree rise in temperature is known as coefficient of volume expansion.
Putting the values of \[{\rho _{t_1}}\] and \[{\rho _{t_2}}\]in the equation:
\[\dfrac{{{\rho _o}}}{{1 + \gamma {t_{_1}}}}.{l_1} = \dfrac{{{\rho _o}}}{{1 + \gamma {t_2}}}.{l_2}\]
Again, cancelling the common terms and cress multiplying the terms:
\[(1 + \gamma {t_2}){l_1} = (1 + \gamma {t_1}){l_2}\]
On further arranging the equation, we arrive at:
\[({l_1} - {l_2}) = \gamma ({l_2}{t_1} - {l_1}{t_2})\]
Thus,
\[\gamma = {\dfrac{{{l_1} - {l_2}}}{{{l_2}{t_1} - {l_1}t}}_2}\]
Therefore, option (B) is the correct answer.
Note: In case of U-tube, when positive pressure is applied in one leg of the U-tube, the liquid level in that particular limb decreases whereas the liquid level in the opposite level increases. U-tubes are widely used to measure pressure losses and difference in pressure in an industrial level. Usually mercury is used to measure pressure difference with U-tube manometers.
Complete step by step solution:
In a U-tube, when pressure is applied the height of liquid at the two limbs are unequal.
Let us consider, in the beginning that the pressure in both the limbs are equal:
Thus, the pressure in the limbs are balanced, so we write:
\[{p_o} + {\rho _{t_2}}g{l_2} = {p_o} + {\rho _{t_1}}g{l_1}\]
Where,
\[{p_o} = \] Atmospheric Pressure.
\[{\rho _{t_2}} = \]Density of liquid at temperature\[{t_2}\].
\[g = \] Acceleration due to gravity.
\[{l_2} = \]Length of second limb.
\[{\rho _{t_1}} = \] Density of liquid at \[{t_2}\].
\[{l_1} = \] Length of first limb.
On cancelling the common terms from sides:
\[{\rho _{t_2}}{l_2} = {\rho _{t1}}{l_1}\]
We know, due to change in temperature, the density of the liquid changes.
Now, let us consider \[{\rho _o}\] to be the initial density of the liquid.
Now, applying the formula that equates density to temperature, we know:
\[{\rho _{t_2}} = \dfrac{{{\rho _o}}}{{1 + \gamma {t_{_2}}}}\]
And, \[{\rho _{t_1}} = \dfrac{{{\rho _o}}}{{1 + \gamma {t_{_1}}}}\]
Where, \[\gamma = \] Coefficient of volume expansion.
When there is a change in volume of a liquid on application of heat (rise or reduction in temperature), the ratio of increase in volume with respect to per degree rise in temperature is known as coefficient of volume expansion.
Putting the values of \[{\rho _{t_1}}\] and \[{\rho _{t_2}}\]in the equation:
\[\dfrac{{{\rho _o}}}{{1 + \gamma {t_{_1}}}}.{l_1} = \dfrac{{{\rho _o}}}{{1 + \gamma {t_2}}}.{l_2}\]
Again, cancelling the common terms and cress multiplying the terms:
\[(1 + \gamma {t_2}){l_1} = (1 + \gamma {t_1}){l_2}\]
On further arranging the equation, we arrive at:
\[({l_1} - {l_2}) = \gamma ({l_2}{t_1} - {l_1}{t_2})\]
Thus,
\[\gamma = {\dfrac{{{l_1} - {l_2}}}{{{l_2}{t_1} - {l_1}t}}_2}\]
Therefore, option (B) is the correct answer.
Note: In case of U-tube, when positive pressure is applied in one leg of the U-tube, the liquid level in that particular limb decreases whereas the liquid level in the opposite level increases. U-tubes are widely used to measure pressure losses and difference in pressure in an industrial level. Usually mercury is used to measure pressure difference with U-tube manometers.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




