
In a set of reactions acetic acid yields a product [D]. The structure of [D] would be:
\[C{H_3}C{H_2}COOH\xrightarrow{{SOC{l_2}}}(A)\xrightarrow[{Anhyd.AlC{l_3}}]{{{C_6}{H_6}}}(B)\xrightarrow{{HCN}}(C)\xrightarrow{{{H_2}O}}\left( D \right)\]
Option:
1)
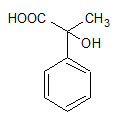
2)
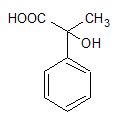
3)
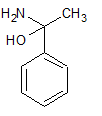
4)
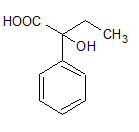
Answer
233.1k+ views
Hint: Think one by one about the product. First think what is the product when carboxylic acid reacts with thionyl chloride $(SOC{l_2})$. After thinking about Friedel-Crafts acylation reaction. Next, think about the product when ketone reacts with HCN. At last, write the product on the reaction between cyanohydrin and ${H_2}O$.
Complete Step by Step Solution:
First make the product A. we all know that carboxylic acid reacts with thionyl chloride $(SOC{l_2})$ and gives acyl chloride as a product. In the reaction the hydroxyl group of the carboxylic acid is changed to a chlorosulfite intermediate which is a better leaving group.Here the nucleophile is chloride anion produced during the reaction .. ${H_2}O$ and $S{O_2}$ are also formed as the by-product.
\[C{H_3}C{H_2}COOH\xrightarrow{{SOC{l_2}}}C{H_3}C{H_2}COCl + {H_2}O + S{O_2}\]
We get the product (A) now let’s find out the product (B). Acyl chloride in presence of anhydrous $AlC{l_3}$ do Friedel-Crafts acylation reaction. In this reaction, one of the hydrogens is substituted by the acyl group on benzene to form acetophenone. Friedel-Crafts acylation reaction is an electrophilic aromatic substitution in which monoacetylated is formed as a product in the reaction between arenes and acyl chlorides or anhydrides. so, the product B is\[C{H_3}C{H_2}COCl\xrightarrow[{Anhyd.AlC{l_3}}]{{{C_6}{H_6}}}{C_6}{H_6}CO{C_2}{H_5}\]
we get product B. Next we will find out product C. Let's do it. A nucleophilic addition reaction happens here. Ketone when react with HCN there is formation of a product called cyanohydrin. So, in this case we also get a cyanohydrin look like

Now we will be going to make our final product that is product D. When water react with cyanohydrin and our final product is
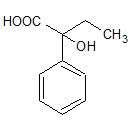
Here the CN group is substituted by the carboxylic acid group.
Therefore, our answer is option D.
Note: When carboxylic acid reacts with thionyl chloride $(SOC{l_2})$ and gives acyl chloride as a product. In Friedel-Crafts acylation reaction in which monoacetylated is formed as a product in the reaction between arenes and acyl chlorides or anhydrides. Ketone and aldehydes when react with HCN are formed cyanohydrin. Water replaces the CN group of cyanohydrins with COOH group.
Complete Step by Step Solution:
First make the product A. we all know that carboxylic acid reacts with thionyl chloride $(SOC{l_2})$ and gives acyl chloride as a product. In the reaction the hydroxyl group of the carboxylic acid is changed to a chlorosulfite intermediate which is a better leaving group.Here the nucleophile is chloride anion produced during the reaction .. ${H_2}O$ and $S{O_2}$ are also formed as the by-product.
\[C{H_3}C{H_2}COOH\xrightarrow{{SOC{l_2}}}C{H_3}C{H_2}COCl + {H_2}O + S{O_2}\]
We get the product (A) now let’s find out the product (B). Acyl chloride in presence of anhydrous $AlC{l_3}$ do Friedel-Crafts acylation reaction. In this reaction, one of the hydrogens is substituted by the acyl group on benzene to form acetophenone. Friedel-Crafts acylation reaction is an electrophilic aromatic substitution in which monoacetylated is formed as a product in the reaction between arenes and acyl chlorides or anhydrides. so, the product B is\[C{H_3}C{H_2}COCl\xrightarrow[{Anhyd.AlC{l_3}}]{{{C_6}{H_6}}}{C_6}{H_6}CO{C_2}{H_5}\]
we get product B. Next we will find out product C. Let's do it. A nucleophilic addition reaction happens here. Ketone when react with HCN there is formation of a product called cyanohydrin. So, in this case we also get a cyanohydrin look like

Now we will be going to make our final product that is product D. When water react with cyanohydrin and our final product is

Here the CN group is substituted by the carboxylic acid group.
Therefore, our answer is option D.
Note: When carboxylic acid reacts with thionyl chloride $(SOC{l_2})$ and gives acyl chloride as a product. In Friedel-Crafts acylation reaction in which monoacetylated is formed as a product in the reaction between arenes and acyl chlorides or anhydrides. Ketone and aldehydes when react with HCN are formed cyanohydrin. Water replaces the CN group of cyanohydrins with COOH group.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




