
If a graph is plotted with the atomic number on x-axis and the square root of the frequency of a spectral line of characteristic X-rays on the y-axis, then it will be
A) A straight line passing through the origin
B) A parabola
C) A straight line not passing through the origin
D) A rectangular hyperbola
Answer
242.1k+ views
Hint: The atomic number or proton number is the number of protons found in the nucleus. A spectral line is a characteristic line in the entire continuous spectrum of an element, resulting from light emission and absorption in a narrow range of frequency when compared with the nearby frequencies.
Complete step by step solution:
The atomic number of an element can be related to the square root of the frequency of a spectral line of characteristic X-rays using Moseley’s law. Moseley’s law is an empirical law concerning the characteristic X-rays emitted by atoms. It states that the square root of the frequency of emitted X-rays is approximately proportional to the atomic number.
Mathematically, the law can be represented using the equation \[\sqrt{\upsilon }\propto Z\] where \[\upsilon \] is the frequency of the emitted X-ray.
The final equation of Moseley’s law can be written as \[\upsilon =A{(Z-b)}^2\] where and are constants depending upon the X-ray emission. Moseley measured and plotted the X-ray frequencies of about 40 elements of the periodic table following his law and observed the graph to be a straight line. The plot of the graph with atomic numbers on the x-axis and the square root of frequencies on the y-axis furnished a straight line not passing through the origin.
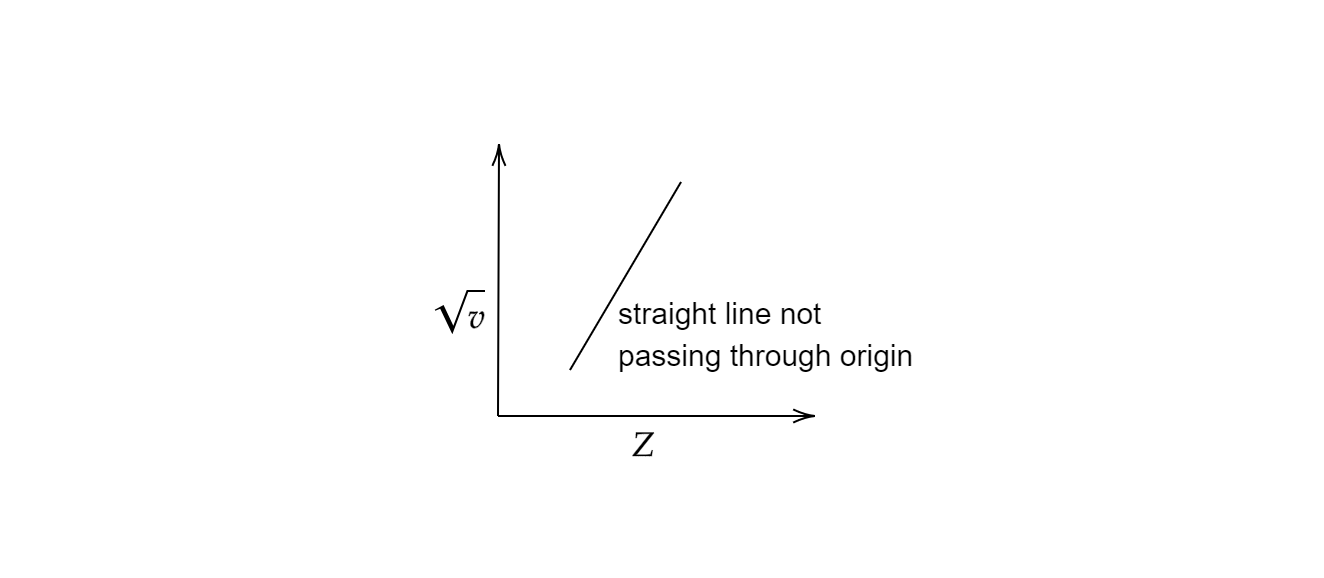
Hence, option (C) is the correct option.
Note: Moseley’s law was the first law to associate the atomic number of elements with any known physical quantity. It established the atomic number as a measurable experimental quantity and also gave it a viable physical meaning. Henry Moseley, the physicist behind this law, has also made major contributions to the periodic table.
Complete step by step solution:
The atomic number of an element can be related to the square root of the frequency of a spectral line of characteristic X-rays using Moseley’s law. Moseley’s law is an empirical law concerning the characteristic X-rays emitted by atoms. It states that the square root of the frequency of emitted X-rays is approximately proportional to the atomic number.
Mathematically, the law can be represented using the equation \[\sqrt{\upsilon }\propto Z\] where \[\upsilon \] is the frequency of the emitted X-ray.
The final equation of Moseley’s law can be written as \[\upsilon =A{(Z-b)}^2\] where and are constants depending upon the X-ray emission. Moseley measured and plotted the X-ray frequencies of about 40 elements of the periodic table following his law and observed the graph to be a straight line. The plot of the graph with atomic numbers on the x-axis and the square root of frequencies on the y-axis furnished a straight line not passing through the origin.

Hence, option (C) is the correct option.
Note: Moseley’s law was the first law to associate the atomic number of elements with any known physical quantity. It established the atomic number as a measurable experimental quantity and also gave it a viable physical meaning. Henry Moseley, the physicist behind this law, has also made major contributions to the periodic table.
Recently Updated Pages
JEE Main 2025-26 Mock Tests: Free Practice Papers & Solutions

JEE Main 2025-26 Experimental Skills Mock Test – Free Practice

JEE Main 2025-26 Electronic Devices Mock Test: Free Practice Online

JEE Main 2025-26 Atoms and Nuclei Mock Test – Free Practice Online

JEE Main 2025-26: Magnetic Effects of Current & Magnetism Mock Test

JEE Main Mock Test 2025: Properties of Solids and Liquids

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Physics Question Paper 2026: Download SET-wise PDF with Answer Key & Analysis

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

CBSE Class 10 Sanskrit Set 4 52 Question Paper 2025 – PDF, Solutions & Analysis

Inductive Effect and Its Role in Acidic Strength




