
Identify meso compounds:
(A) 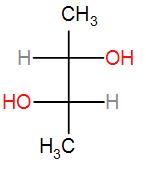
(B) 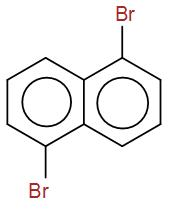
(C) 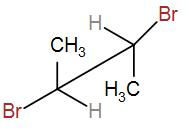
(D) 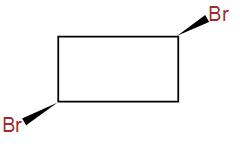
Answer
233.1k+ views
Hint: Meso compounds are compounds that do not show optical activity even though they have chiral centres. They have a plane of symmetry. To solve this, find the plane of symmetry in the given structures and then identify their stereochemistry.
Complete step by step solution:
To answer this question, we have to understand what meso compounds are.
Meso compounds are compounds which have multiple stereocenters i.e. chiral centres but are optically inactive. The chiral centre is an atom to which 4 different groups of atoms are bonded so that they can form a non-superimposable mirror image.
Meso compounds have multiple chiral centres but are achiral.
To identify a meso compound, we can follow the steps below-
- A meso compound will have two or more chiral centres.
- After you have determined the chiral centres, look for an internal plane that can be passed through the compound.
- Then look for the stereochemistry of the compound i.e. determine whether it is R or S. If there are two chiral centres and RS = SR, then it is a meso compound.
Meso compound is optically inactive so the R cancels out S in a meso compound.
Basically, if you can find out a plane of symmetry and two or more chiral centres in a compound then it is a meso compound.
Now, let us go through the options given to us.
Firstly, we have –

If we pass a plane in between and determine the stereochemistry of the two chiral centres, both will come out to be R. So this is not a meso compound.
Then we have-
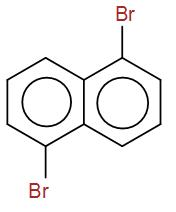
It is not a meso compound as it has no plane of symmetry.
Next, we have-
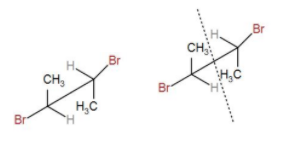
In Fischer projection, we can draw it as-
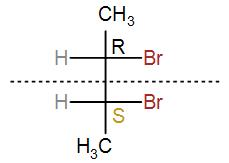
Here, we can see that RS = SR. Therefore, this is a meso compound.
And lastly, we have –
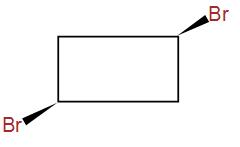
It has a plane of symmetry but it has no chiral centres.
Therefore, the correct answer is option (C).
Note: We know that optical isomers are compounds having the same atoms and bonds but in different spatial arrangements and will have non-superimposable mirror images. However, meso compounds are also optical isomers, but they contain a plane of symmetry. All molecules irrespective of their chirality exhibit optical activity when they are not superimposable on their mirror images.
Complete step by step solution:
To answer this question, we have to understand what meso compounds are.
Meso compounds are compounds which have multiple stereocenters i.e. chiral centres but are optically inactive. The chiral centre is an atom to which 4 different groups of atoms are bonded so that they can form a non-superimposable mirror image.
Meso compounds have multiple chiral centres but are achiral.
To identify a meso compound, we can follow the steps below-
- A meso compound will have two or more chiral centres.
- After you have determined the chiral centres, look for an internal plane that can be passed through the compound.
- Then look for the stereochemistry of the compound i.e. determine whether it is R or S. If there are two chiral centres and RS = SR, then it is a meso compound.
Meso compound is optically inactive so the R cancels out S in a meso compound.
Basically, if you can find out a plane of symmetry and two or more chiral centres in a compound then it is a meso compound.
Now, let us go through the options given to us.
Firstly, we have –

If we pass a plane in between and determine the stereochemistry of the two chiral centres, both will come out to be R. So this is not a meso compound.
Then we have-

It is not a meso compound as it has no plane of symmetry.
Next, we have-

In Fischer projection, we can draw it as-

Here, we can see that RS = SR. Therefore, this is a meso compound.
And lastly, we have –

It has a plane of symmetry but it has no chiral centres.
Therefore, the correct answer is option (C).
Note: We know that optical isomers are compounds having the same atoms and bonds but in different spatial arrangements and will have non-superimposable mirror images. However, meso compounds are also optical isomers, but they contain a plane of symmetry. All molecules irrespective of their chirality exhibit optical activity when they are not superimposable on their mirror images.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




