
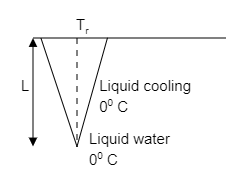
Icicles. Liquid water coats an active (growing) icicle and extends up a short, narrow tube along the central axis. Because the water ice interface must have a temperature of ${0^ \circ }\,C $, the water in the tube cannot lose energy through the sides of the icicle or down through the tip because there are no temperature changes in those directions. It can lose energy and freeze only by sending energy up through distance $L $ to the top of the icicle, where the temperature ${T_r} $ can be below ${0^ \circ }\,C $. Take $L = 0.12\,m $ and ${T_r} = - {5^ \circ }\,C $. Assume that the central tube and the upward conduction path both have cross sectional area $A $. In terms of $A $, what rate is mass converted from liquid to ice at the top of the central tube?
Answer
240.6k+ views
Hint The rate of the mass converted from liquid to the ice at the top of the central tube can be determined by using the heat of fusion formula and this formula is differentiated with respect to the time, then the rate of the mass converted from liquid to ice can be determined.
Useful formula
The heat of the fusion can be given by,
$Q = {L_F}m $
Where, $Q $ is the heat of the fusion, ${L_F} $ is the constant and $m $ is the mass.
Complete step by step answer
Given that,
The length of the tube is, $L = 0.12\,m $,
The temperature of the liquid is, ${T_r} = - {5^ \circ }\,C $.
Now,
The heat of the fusion can be given by,
$Q = {L_F}m $
By differentiating the above equation with respect to the time, then the above equation is written as,
$\dfrac{{dQ}}{{dt}} = {L_F}\dfrac{{dm}}{{dt}} $
The above equation is also written as,
${P_{cond}} = \dfrac{{dQ}}{{dt}} = {L_F}\dfrac{{dm}}{{dt}} $
By rearranging the terms in the above equation, then the above equation is written as,
$\dfrac{{{P_{cond}}}}{{{L_F}}} = \dfrac{{dm}}{{dt}} $
The value of the ${P_{cond}} = 16.7\,AW $ and the value of the ${L_F} = 3.33 \times {10^5}\,Jk{g^{ - 1}} $.
By substituting the both values in the above equation, then the above equation is written as,
$\dfrac{{dm}}{{dt}} = \dfrac{{16.7\,AW}}{{3.33 \times {{10}^5}\,Jk{g^{ - 1}}}} $
By dividing the terms in the above equation, then the above equation is written as,
$\dfrac{{dm}}{{dt}} = 5.02 \times {10^{ - 5}}\,kg{s^{ - 1}} $
Thus, the above equation shows the rate is mass converted from liquid to ice at the top of the central tube.
Note The rate of the mass of the liquid converted to the ice is directly proportional to the ${P_{cond}} $ and the rate of the mass of the liquid converted to the ice is inversely proportional to the ${L_F} $. As the ${P_{cond}} $ increases, the rate of the mass of the liquid converted to the ice also increases.
Useful formula
The heat of the fusion can be given by,
$Q = {L_F}m $
Where, $Q $ is the heat of the fusion, ${L_F} $ is the constant and $m $ is the mass.
Complete step by step answer
Given that,
The length of the tube is, $L = 0.12\,m $,
The temperature of the liquid is, ${T_r} = - {5^ \circ }\,C $.
Now,
The heat of the fusion can be given by,
$Q = {L_F}m $
By differentiating the above equation with respect to the time, then the above equation is written as,
$\dfrac{{dQ}}{{dt}} = {L_F}\dfrac{{dm}}{{dt}} $
The above equation is also written as,
${P_{cond}} = \dfrac{{dQ}}{{dt}} = {L_F}\dfrac{{dm}}{{dt}} $
By rearranging the terms in the above equation, then the above equation is written as,
$\dfrac{{{P_{cond}}}}{{{L_F}}} = \dfrac{{dm}}{{dt}} $
The value of the ${P_{cond}} = 16.7\,AW $ and the value of the ${L_F} = 3.33 \times {10^5}\,Jk{g^{ - 1}} $.
By substituting the both values in the above equation, then the above equation is written as,
$\dfrac{{dm}}{{dt}} = \dfrac{{16.7\,AW}}{{3.33 \times {{10}^5}\,Jk{g^{ - 1}}}} $
By dividing the terms in the above equation, then the above equation is written as,
$\dfrac{{dm}}{{dt}} = 5.02 \times {10^{ - 5}}\,kg{s^{ - 1}} $
Thus, the above equation shows the rate is mass converted from liquid to ice at the top of the central tube.
Note The rate of the mass of the liquid converted to the ice is directly proportional to the ${P_{cond}} $ and the rate of the mass of the liquid converted to the ice is inversely proportional to the ${L_F} $. As the ${P_{cond}} $ increases, the rate of the mass of the liquid converted to the ice also increases.
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Physics Chapter 4 - Laws of Motion - 2025-26

CBSE Notes Class 11 Physics Chapter 14 - Waves - 2025-26

CBSE Notes Class 11 Physics Chapter 9 - Mechanical Properties of Fluids - 2025-26

Inductive Effect and Its Role in Acidic Strength




