
_________ grams of 3-Hydroxy propanal (MW=74) must be dehydrated to produce 7.8g of acrolein (MW=56) (\[{C_3}{H_4}O\]) if the percentage yield is 64. (Round off to the nearest integer).
[Given atomic masses; C=12.0 \[\mu \], H=1.0\[\mu \] , O=16.0\[\mu \]]
Answer
233.1k+ views
Hint: In the above-mentioned chemical reaction dehydration reaction is taking place. A dehydration reaction is a type of reaction in which there will be a loss of water from the chemical reaction. It is the reverse process of hydration reaction.
Complete Step by Step Solution:
When 3-Hydroxy propanal is dehydrated to produce 7.8g of acrolein, the chemical reaction is given as:
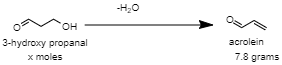
The moles of acrolein produced in the chemical reaction is:
\[\dfrac{{7.8}}{{56}} = 0.14\] moles
The percentage of yield would be equal to
\[\dfrac{{7.8/56}}{x} \times 100 = 64\]
\[x = \dfrac{{7.8 \times 100}}{{56 \times 64}} = \dfrac{{780}}{{56 \times 64}}\] moles
\[{W_{reac\tan t}} = \dfrac{{780}}{{56 \times 64}} \times 74 = 16.11\] grams
Therefore, 16.11 grams of 3-hydroxy propanal must be dehydrated to produce 7.8 grams of acrolein.
Note: Acrolein usually occurs as a colourless or yellow-colored liquid that dissolves in water very easily. It also has the tendency to quickly change into a vapour state when it is heated. Acrolein has a wide variety of uses such as in pesticides for controlling algae, weeds, bacteria, and mollusks. It also helps in the manufacturing of other industrial-based chemicals. It has a very choking odour and causes severe irritation to the eyes and mucous membrane. It is also toxic when it is inhaled and has a flash point which is below \[{0^o}F\]. It is less dense than water and its vapours are heavier than air. Very small amounts of acrolein can be produced and enter the atmosphere when oil. Plastics. Tobacco, gasoline, and oil are burned.
Complete Step by Step Solution:
When 3-Hydroxy propanal is dehydrated to produce 7.8g of acrolein, the chemical reaction is given as:

The moles of acrolein produced in the chemical reaction is:
\[\dfrac{{7.8}}{{56}} = 0.14\] moles
The percentage of yield would be equal to
\[\dfrac{{7.8/56}}{x} \times 100 = 64\]
\[x = \dfrac{{7.8 \times 100}}{{56 \times 64}} = \dfrac{{780}}{{56 \times 64}}\] moles
\[{W_{reac\tan t}} = \dfrac{{780}}{{56 \times 64}} \times 74 = 16.11\] grams
Therefore, 16.11 grams of 3-hydroxy propanal must be dehydrated to produce 7.8 grams of acrolein.
Note: Acrolein usually occurs as a colourless or yellow-colored liquid that dissolves in water very easily. It also has the tendency to quickly change into a vapour state when it is heated. Acrolein has a wide variety of uses such as in pesticides for controlling algae, weeds, bacteria, and mollusks. It also helps in the manufacturing of other industrial-based chemicals. It has a very choking odour and causes severe irritation to the eyes and mucous membrane. It is also toxic when it is inhaled and has a flash point which is below \[{0^o}F\]. It is less dense than water and its vapours are heavier than air. Very small amounts of acrolein can be produced and enter the atmosphere when oil. Plastics. Tobacco, gasoline, and oil are burned.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




