
For the reaction, \[aA + bB\xrightarrow{{}}cC + dD\] , the plot of \[\log k\] vs \[1/T\] is given below:
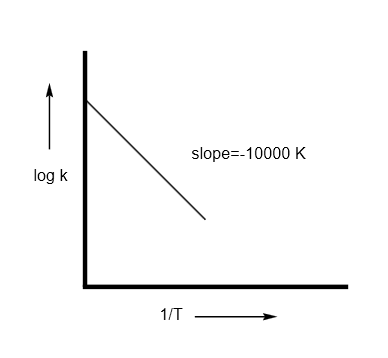
The temperature at which the rate constant of the reaction is \[{10^{ - 4}}{s^{ - 1}}\] is ______ \[K\] . [Rounded off to the nearest integer]
[given: the rate constant of the reaction is \[{10^{ - 5}}{s^{ - 1}}\] at \[500\,K\] ].
Answer
232.8k+ views
Hint: Here, in this question, a graph of \[\log k\] vs \[1/T\] is given. We have to use the Arrhenius equation for calculating the temperature.
Formula Used:
The Arrhenius equation is as follows:
\[k = A{e^{ - \dfrac{{{E_a}}}{{RT}}}}\]
Here, \[k\] is the rate constant of the reaction, \[A\] is the pre-exponential factor, \[e\] is the base of natural logarithm (Euler’s number), \[{E_a}\] is the activation energy of the chemical reaction in terms of energy per mole, \[R\] is universal gas constant, \[T\] is the absolute temperature associated with the reaction (in Kelvin).
Complete Step by Step Solution:
The Arrhenius equation is a formula that shows how the rate constant (of a chemical reaction), absolute temperature, and the A factor are related (also known as the pre-exponential factor; can be visualised as the frequency of correctly oriented collisions between reactant particles). It elucidates the relationship between response rates and temperature absolute.
From the Arrhenius equation, let us calculate the temperature as follows:
$ k = A{e^{ - \dfrac{{{E_a}}}{{RT}}}} \\$
$ \ln k = \ln A - \dfrac{{{E_a}}}{{RT}} \\$
$ 2.303 \times \log k = \left( {2.303 \times \log A} \right) - \dfrac{{{E_a}}}{{RT}} \\ $
Further solving,
\[\log k = \log A - \dfrac{{{E_a}}}{{2.303RT}}\]
Now, compare the above equation with the common equation of line \[y = mx + c\].
We get the value of slope,
$ slope = - \dfrac{{{E_a}}}{{2.303RT}} \\$
$ \Rightarrow - \dfrac{{{E_a}}}{{2.303RT}} = - 10000 \\$
$ \Rightarrow \dfrac{{{E_a}}}{{2.303RT}} = 10000 \\ $
From the Arrhenius equation,
$ \log \dfrac{{{k_1}}}{{{k_2}}} = \dfrac{{{E_a}}}{{2.303R}} \times \left( {\dfrac{1}{{{T_1}}} - \dfrac{1}{{{T_2}}}} \right) \\$
$ \Rightarrow \log \dfrac{{{{10}^{ - 4}}}}{{{{10}^{ - 5}}}} = 10000 \times \left( {\dfrac{1}{{500}} - \dfrac{1}{{{T_{}}}}} \right) \\$
$ \Rightarrow 1 = 10000 \times \left( {\dfrac{1}{{500}} - \dfrac{1}{{{T_{}}}}} \right) \\ $
Further solving,
$ \dfrac{1}{{10000}} = \dfrac{1}{{500}} - \dfrac{1}{T} \\$
$ \Rightarrow \dfrac{1}{T} = \dfrac{1}{{500}} - \dfrac{1}{{10000}} \\$
$ \Rightarrow \dfrac{1}{T} = \dfrac{{20 - 1}}{{10000}} \\ $
Further solving,
$ \dfrac{1}{T} = \dfrac{{19}}{{10000}} \\$
$ \Rightarrow T = \dfrac{{10000}}{{19}} \\$
$ \Rightarrow T = 526\,K \\ $
As a result, the temperature is \[526\,K\] at which the rate constant of the reaction is \[{10^{ - 4}}{s^{ - 1}}\] .
Note: Catalysts aid in decreasing the activation energy required for a specific reaction. As a result, the decreased activation energy accounted for by catalysts is substituted in the Arrhenius equation to obtain the catalysed reaction rate constant.
Formula Used:
The Arrhenius equation is as follows:
\[k = A{e^{ - \dfrac{{{E_a}}}{{RT}}}}\]
Here, \[k\] is the rate constant of the reaction, \[A\] is the pre-exponential factor, \[e\] is the base of natural logarithm (Euler’s number), \[{E_a}\] is the activation energy of the chemical reaction in terms of energy per mole, \[R\] is universal gas constant, \[T\] is the absolute temperature associated with the reaction (in Kelvin).
Complete Step by Step Solution:
The Arrhenius equation is a formula that shows how the rate constant (of a chemical reaction), absolute temperature, and the A factor are related (also known as the pre-exponential factor; can be visualised as the frequency of correctly oriented collisions between reactant particles). It elucidates the relationship between response rates and temperature absolute.
From the Arrhenius equation, let us calculate the temperature as follows:
$ k = A{e^{ - \dfrac{{{E_a}}}{{RT}}}} \\$
$ \ln k = \ln A - \dfrac{{{E_a}}}{{RT}} \\$
$ 2.303 \times \log k = \left( {2.303 \times \log A} \right) - \dfrac{{{E_a}}}{{RT}} \\ $
Further solving,
\[\log k = \log A - \dfrac{{{E_a}}}{{2.303RT}}\]
Now, compare the above equation with the common equation of line \[y = mx + c\].
We get the value of slope,
$ slope = - \dfrac{{{E_a}}}{{2.303RT}} \\$
$ \Rightarrow - \dfrac{{{E_a}}}{{2.303RT}} = - 10000 \\$
$ \Rightarrow \dfrac{{{E_a}}}{{2.303RT}} = 10000 \\ $
From the Arrhenius equation,
$ \log \dfrac{{{k_1}}}{{{k_2}}} = \dfrac{{{E_a}}}{{2.303R}} \times \left( {\dfrac{1}{{{T_1}}} - \dfrac{1}{{{T_2}}}} \right) \\$
$ \Rightarrow \log \dfrac{{{{10}^{ - 4}}}}{{{{10}^{ - 5}}}} = 10000 \times \left( {\dfrac{1}{{500}} - \dfrac{1}{{{T_{}}}}} \right) \\$
$ \Rightarrow 1 = 10000 \times \left( {\dfrac{1}{{500}} - \dfrac{1}{{{T_{}}}}} \right) \\ $
Further solving,
$ \dfrac{1}{{10000}} = \dfrac{1}{{500}} - \dfrac{1}{T} \\$
$ \Rightarrow \dfrac{1}{T} = \dfrac{1}{{500}} - \dfrac{1}{{10000}} \\$
$ \Rightarrow \dfrac{1}{T} = \dfrac{{20 - 1}}{{10000}} \\ $
Further solving,
$ \dfrac{1}{T} = \dfrac{{19}}{{10000}} \\$
$ \Rightarrow T = \dfrac{{10000}}{{19}} \\$
$ \Rightarrow T = 526\,K \\ $
As a result, the temperature is \[526\,K\] at which the rate constant of the reaction is \[{10^{ - 4}}{s^{ - 1}}\] .
Note: Catalysts aid in decreasing the activation energy required for a specific reaction. As a result, the decreased activation energy accounted for by catalysts is substituted in the Arrhenius equation to obtain the catalysed reaction rate constant.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




