
Fluorine is prepared by
A. Oxidation of $HF$
B. Electrolysis of $KF$
C. Electrolysis of fused $KH{F_2}$
D. Decomposition of $Hg{F_2}$
Answer
233.1k+ views
Hint: Florine is prepared by using the famous electrolysis method called Whytlaw-Grey method based on the idea that $KH{F_2}$, which is dry, anhydrous, and pure in nature, is electrolyzed while it is still molten. Here, we can observe that hydrogen and fluorine are released at the cathode and anode, respectively.
Complete Step by Step Solution:
In electric warmed copper cells that also serve as the cathode, electrolysis of fused potassium hydrogen fluoride is conducted.
$2KH{F_2} \to 2KF + 2HF$
$2KF \to 2{K^ + } + 2{F^ - }$
It causes the following reaction to occur at the anode after additional electrolysis:
$2{F^ - } \to {F_2} + 2{e^ - }$
So, this is the fluorine we want.
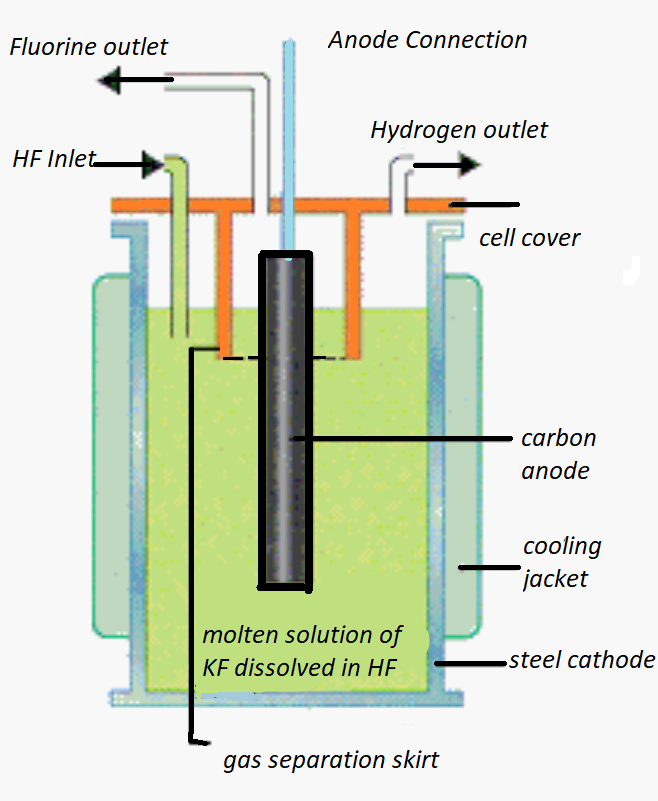
If we look at the diagram, we can see that it consists of a heated copper cell, coils, a solution that has been fused with $KH{F_2}$ , a graphite anode, a copper diaphragm, a fluorspar stopper, etc.
There is a cylindrical vessel with vertical walls that prevent electrolyte creep. Additionally, there is a diaphragm in place between the electrodes, which essentially stops hydrogen and fluorine from mingling. There are electrodes; from the diagram, we can see that the copper vessel serves as the cathode and the graphite rod serves as the anode.
We can observe that a diaphragm separates the anode and cathode. A fluorspar paste is discovered to be isolating the anode from the diaphragm cell. The non-porous upper portion leads to an ${F_2}$ gas supply tube.
It can also be noted that the $KH{F_2}$ electrolyte, which is dry, pure, and anhydrous in nature, is employed during the electrolysis process, where the temperature is likewise kept at around 523K.
The inner copper diaphragm has a fluorine gas outlet, while the outer copper vessel has a hydrogen gas outlet. The fluorine gas is then compressed and stored in Cu-Ni cylinders after being collected in copper cylinders.
Hence the correct option is C.
Note: Graphite is not a suitable electrode as anode because it combines with Fluorine and forms $C{F_4}$. Hence it is necessary to replace the graphite electrode from time to time. Also, we can state that this approach is more effective than other procedures, and the effectiveness at present time is up to 80%. Additionally, this procedure is ongoing.
Complete Step by Step Solution:
In electric warmed copper cells that also serve as the cathode, electrolysis of fused potassium hydrogen fluoride is conducted.
$2KH{F_2} \to 2KF + 2HF$
$2KF \to 2{K^ + } + 2{F^ - }$
It causes the following reaction to occur at the anode after additional electrolysis:
$2{F^ - } \to {F_2} + 2{e^ - }$
So, this is the fluorine we want.

If we look at the diagram, we can see that it consists of a heated copper cell, coils, a solution that has been fused with $KH{F_2}$ , a graphite anode, a copper diaphragm, a fluorspar stopper, etc.
There is a cylindrical vessel with vertical walls that prevent electrolyte creep. Additionally, there is a diaphragm in place between the electrodes, which essentially stops hydrogen and fluorine from mingling. There are electrodes; from the diagram, we can see that the copper vessel serves as the cathode and the graphite rod serves as the anode.
We can observe that a diaphragm separates the anode and cathode. A fluorspar paste is discovered to be isolating the anode from the diaphragm cell. The non-porous upper portion leads to an ${F_2}$ gas supply tube.
It can also be noted that the $KH{F_2}$ electrolyte, which is dry, pure, and anhydrous in nature, is employed during the electrolysis process, where the temperature is likewise kept at around 523K.
The inner copper diaphragm has a fluorine gas outlet, while the outer copper vessel has a hydrogen gas outlet. The fluorine gas is then compressed and stored in Cu-Ni cylinders after being collected in copper cylinders.
Hence the correct option is C.
Note: Graphite is not a suitable electrode as anode because it combines with Fluorine and forms $C{F_4}$. Hence it is necessary to replace the graphite electrode from time to time. Also, we can state that this approach is more effective than other procedures, and the effectiveness at present time is up to 80%. Additionally, this procedure is ongoing.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




