
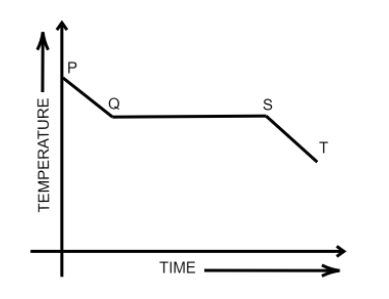
Fig. shows the variation in temperature with time when some wax cools from the liquid phase to the solid phase. In which part of the curve, the wax will be in the liquid as well as in the solid phase?
(A) PQ
(B) QS
(C) ST
(D) Can’t say
Answer
232.8k+ views
Hint As the temperature of the wax lowers, its state changes from a liquid state to a solid-state. The slope of the curve when the temperature is lowered is non-zero, but when the phase transformation occurs, the temperature doesn’t change with respect to time until all liquid wax is converted into solid wax, therefore the slope of the curve at this stage is zero.
Complete Step by step solution
During the cooling of wax, heat is constantly transferred to the environment resulting in the cooling of the wax. After a certain amount of heat is given off, the wax “freezes” or converts into a solid-state.
Phase transformation is a process when all the heat that is given off is used to convert the phase of the wax from liquid to solid. Thus during this process, the temperature of the wax remains constant.
During the phase transformation, the wax exists in both the solid and the liquid state.
From the diagram, it can be said that-
From P to Q the temperature of the liquid wax drops. The slope of temperature versus time decreases.
At Q the phase transformation starts.
From Q to S the phase transforms from liquid to solid, known as “freezing”. The wax exists in both solid and liquid states. Here the slope of temperature versus time graph is zero.
At S all of the liquid wax is transformed into solid, or the wax is said to be “frozen”.
From S to Q the temperature of the solid wax decreases further as it continues to lose heat.
Therefore, the correct option is (B).
Note During phase transformation, all the heat given to the system or lost by the system is used up to change the state of the substance. This heat is also known as “latent heat” it is used as a method to reduce the temperature in certain cooling mechanisms.
Complete Step by step solution
During the cooling of wax, heat is constantly transferred to the environment resulting in the cooling of the wax. After a certain amount of heat is given off, the wax “freezes” or converts into a solid-state.
Phase transformation is a process when all the heat that is given off is used to convert the phase of the wax from liquid to solid. Thus during this process, the temperature of the wax remains constant.
During the phase transformation, the wax exists in both the solid and the liquid state.
From the diagram, it can be said that-
From P to Q the temperature of the liquid wax drops. The slope of temperature versus time decreases.
At Q the phase transformation starts.
From Q to S the phase transforms from liquid to solid, known as “freezing”. The wax exists in both solid and liquid states. Here the slope of temperature versus time graph is zero.
At S all of the liquid wax is transformed into solid, or the wax is said to be “frozen”.
From S to Q the temperature of the solid wax decreases further as it continues to lose heat.
Therefore, the correct option is (B).
Note During phase transformation, all the heat given to the system or lost by the system is used up to change the state of the substance. This heat is also known as “latent heat” it is used as a method to reduce the temperature in certain cooling mechanisms.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




