
Explain the following name reaction with a suitable chemical reaction.
Cannizaro’s reaction.
Answer
232.8k+ views
Hint: This reaction is named after its discoverer Stanislao Cannizzaro. You should know that the products formed from this reaction are primary alcohol and a carboxylic acid. Now try solving the question.
Complete step by step answer:
> Let us know this reaction in detail.
The Cannizzaro reaction is a redox reaction in which two molecules of an aldehyde are reacted to produce a primary alcohol and a carboxylic acid using a hydroxide base.
> Let us see the mechanism of Cannizzaro reaction.
- You should know that the reaction begins with hydroxide attack on the carbonyl carbon followed by deprotonation to give a dianion. This unstable intermediate releases a hydride anion which attacks another molecule of aldehyde.
- In this process the dianion converts to a carboxylate anion and the aldehyde to an alkoxide.
The alkoxide in the reaction then picks up a proton from water to provide the alcohol as the final product, while the carboxylate is converted to the carboxylic acid product after acid work-up.
Let us understand better with the help of the reaction.
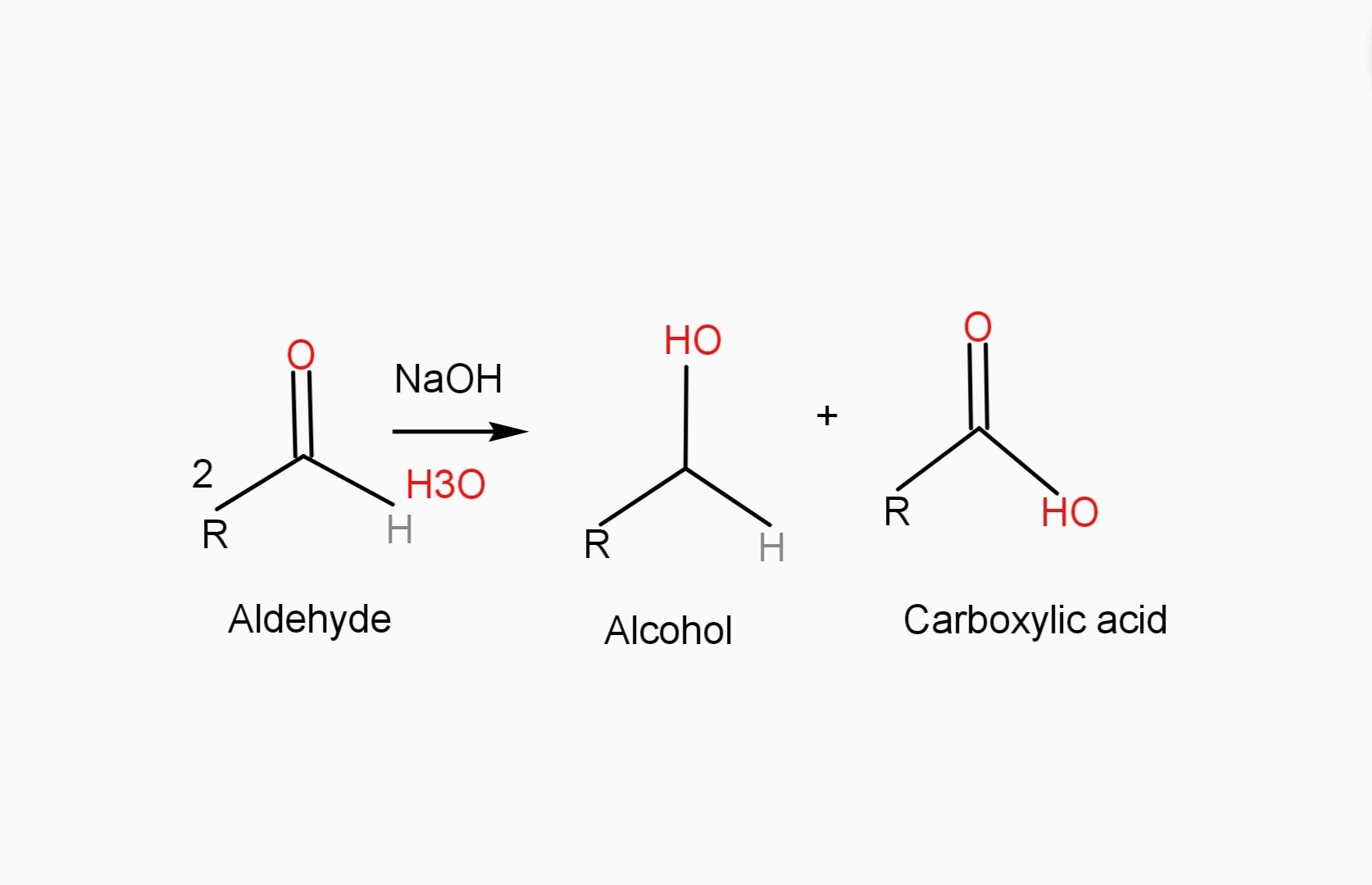
Example of Cannizzaro reaction , when formaldehyde is disproportionated to formic acid and methyl alcohol in strong alkali.
$HCHO\xrightarrow [ NaOH ]{ } HCO{ O }^{ - }{ Na }^{ + }+{ CH }_{ 3 }OH$
Note: The variations of the reaction improve the yield of the desired product. The Cannizzaro reaction can be used to influence a disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
Complete step by step answer:
> Let us know this reaction in detail.
The Cannizzaro reaction is a redox reaction in which two molecules of an aldehyde are reacted to produce a primary alcohol and a carboxylic acid using a hydroxide base.
> Let us see the mechanism of Cannizzaro reaction.
- You should know that the reaction begins with hydroxide attack on the carbonyl carbon followed by deprotonation to give a dianion. This unstable intermediate releases a hydride anion which attacks another molecule of aldehyde.
- In this process the dianion converts to a carboxylate anion and the aldehyde to an alkoxide.
The alkoxide in the reaction then picks up a proton from water to provide the alcohol as the final product, while the carboxylate is converted to the carboxylic acid product after acid work-up.
Let us understand better with the help of the reaction.

Example of Cannizzaro reaction , when formaldehyde is disproportionated to formic acid and methyl alcohol in strong alkali.
$HCHO\xrightarrow [ NaOH ]{ } HCO{ O }^{ - }{ Na }^{ + }+{ CH }_{ 3 }OH$
Note: The variations of the reaction improve the yield of the desired product. The Cannizzaro reaction can be used to influence a disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




