
Ethylene oxide when treated with Grignard reagent yields:
(A) Secondary alcohol
(B) Tertiary alcohol
(C) Cyclopropyl alcohol
(D) Primary alcohol
Answer
240.3k+ views
Hint: Ethylene oxide is a very reactive molecule and its C-O bond will get cleaved as nucleophilic carbon atom of Grignard reagent will attack on the electrophilic carbon atom of ethylene oxide. Ethylene oxide is also called oxirane.
Step by step answer:
- Ethylene oxide is also known by another name which is oxirane. We are taking the general Grignard reagent which is RMgX.
- Grignard reagents are a group of reagents, which are generated by reacting organic halides and magnesium metal (Mg) mostly in the presence of ether. They have the general formula RMgX. Here, R represents an organic group (alkyl or alkenyl) while X represents a halogen. They are applied in the Grignard reaction.
The reaction of Grignard reagent with ethylene oxide is represented below.
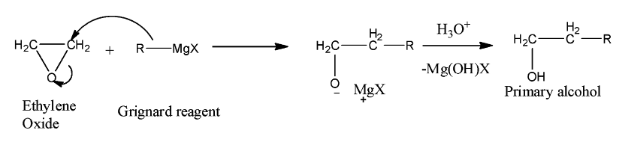
- We can see that the nucleophilic alkyl group of Grignard reagent will attack the electrophilic atom of ethylene oxide which is carbon. So, C-O bond of ethylene oxide will get broken and an organo-magnesium compound will form. Then, upon hydrolysis, we will get alcohol as a product.
- In the product molecule, the hydroxyl group is attached to a carbon that is bonded with other one carbon atom. So, we can say that alcohol is a primary alcohol.
Thus, we can conclude that Ethylene oxide reacts with Grignard reagent to give a primary alcohol.
So, option (D) is a correct answer.
Note: We should note that, when we are performing a reaction involving Grignard reagents, it is necessary to ensure that no water is present which would otherwise cause the reagent to decompose rapidly. Therefore, the majority of Grignard reactions occur in solvents such as anhydrous diethyl ether.
Step by step answer:
- Ethylene oxide is also known by another name which is oxirane. We are taking the general Grignard reagent which is RMgX.
- Grignard reagents are a group of reagents, which are generated by reacting organic halides and magnesium metal (Mg) mostly in the presence of ether. They have the general formula RMgX. Here, R represents an organic group (alkyl or alkenyl) while X represents a halogen. They are applied in the Grignard reaction.
The reaction of Grignard reagent with ethylene oxide is represented below.

- We can see that the nucleophilic alkyl group of Grignard reagent will attack the electrophilic atom of ethylene oxide which is carbon. So, C-O bond of ethylene oxide will get broken and an organo-magnesium compound will form. Then, upon hydrolysis, we will get alcohol as a product.
- In the product molecule, the hydroxyl group is attached to a carbon that is bonded with other one carbon atom. So, we can say that alcohol is a primary alcohol.
Thus, we can conclude that Ethylene oxide reacts with Grignard reagent to give a primary alcohol.
So, option (D) is a correct answer.
Note: We should note that, when we are performing a reaction involving Grignard reagents, it is necessary to ensure that no water is present which would otherwise cause the reagent to decompose rapidly. Therefore, the majority of Grignard reactions occur in solvents such as anhydrous diethyl ether.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26




