
Ethyl methylamine cannot be resolved under normal conditions because
A. The nitrogen atom rapidly inverts its configuration leading to a mixture.
B. It isomerizes rapidly with the achiral isomer trimethylamine.
C. The favored configuration is not chiral.
D. The C-N bond is not stable under conditions used for resolution.
Answer
232.8k+ views
Hint: Those amines are chiral which have three different groups with tetrahedral configurations. Their enantiomeric forms of chiral amines can be resolved under normal conditions. Because they undergo pyramidal inversion. To answer this question, first, we have to check whether ethyl methylamine is a chiral or achiral compound.
Complete step by step solution:
Secondary and tertiary amines with three different groups bonded to nitrogen are generally chiral compounds that can not be easily resolved at room temperature. Because nitrogen inversion or pyramidal inversion rapidly inverts the two enantiomers.
We have ethyl methylamine which is a chiral compound as the nitrogen center has four different groups (-Et, -Me, –H group and a lone pair of electrons) in other words it has non-superimposable mirror images. The structure of chiral ethyl methylamine is shown below:
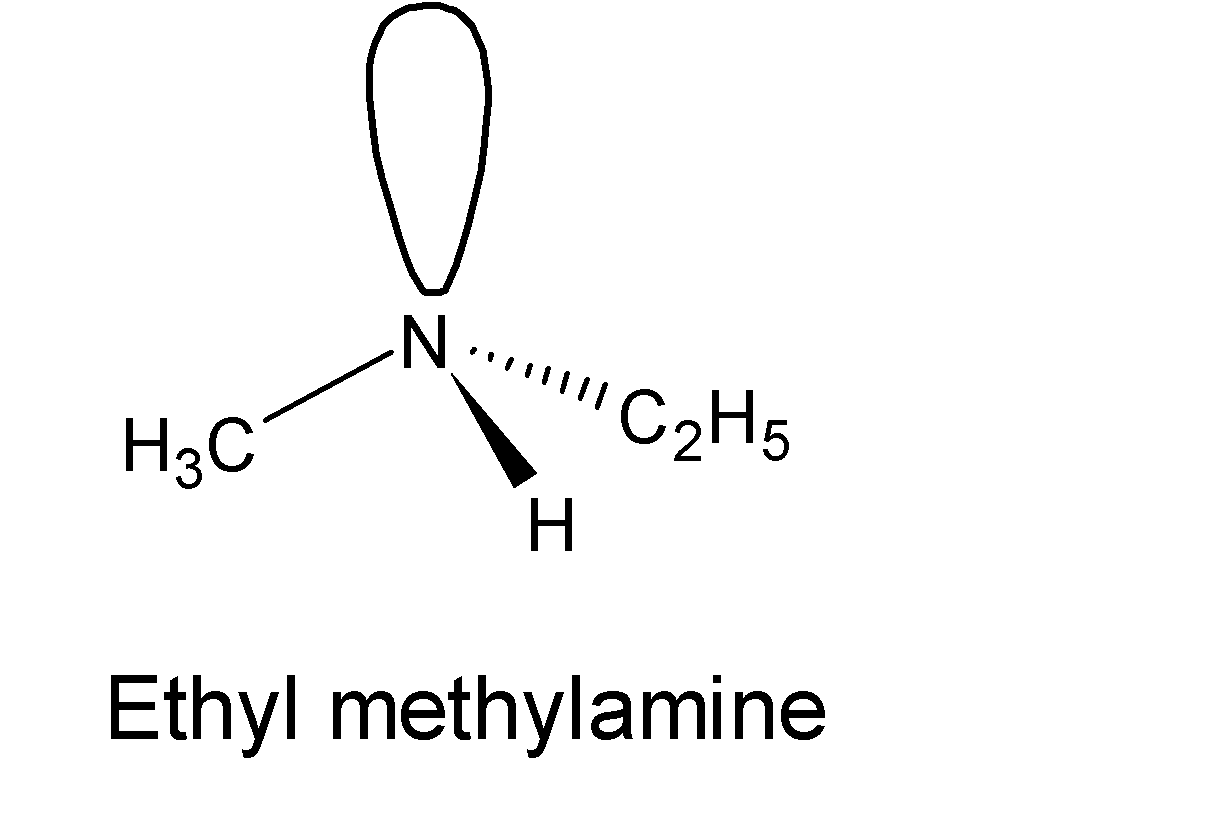
Nitrogen atom bonded with three groups possesses $s{{p}^{3}}$ hybridization and trigonal pyramidal geometry. At room temperature, the two arrangements of ethyl methylamine are in equilibrium and rapidly invert its stereochemistry via trigonal planar,$s{{p}^{2}}$ nitrogen atom where the lone pair of electrons lie in the unhybridized $2p$ orbital. During this inversion, there is a rapid oscillation of nitrogen atoms from one side of the plane to another side of the plane. Like this nitrogen completes its inversion process and back to its $s{{p}^{3}}$ hybridization.
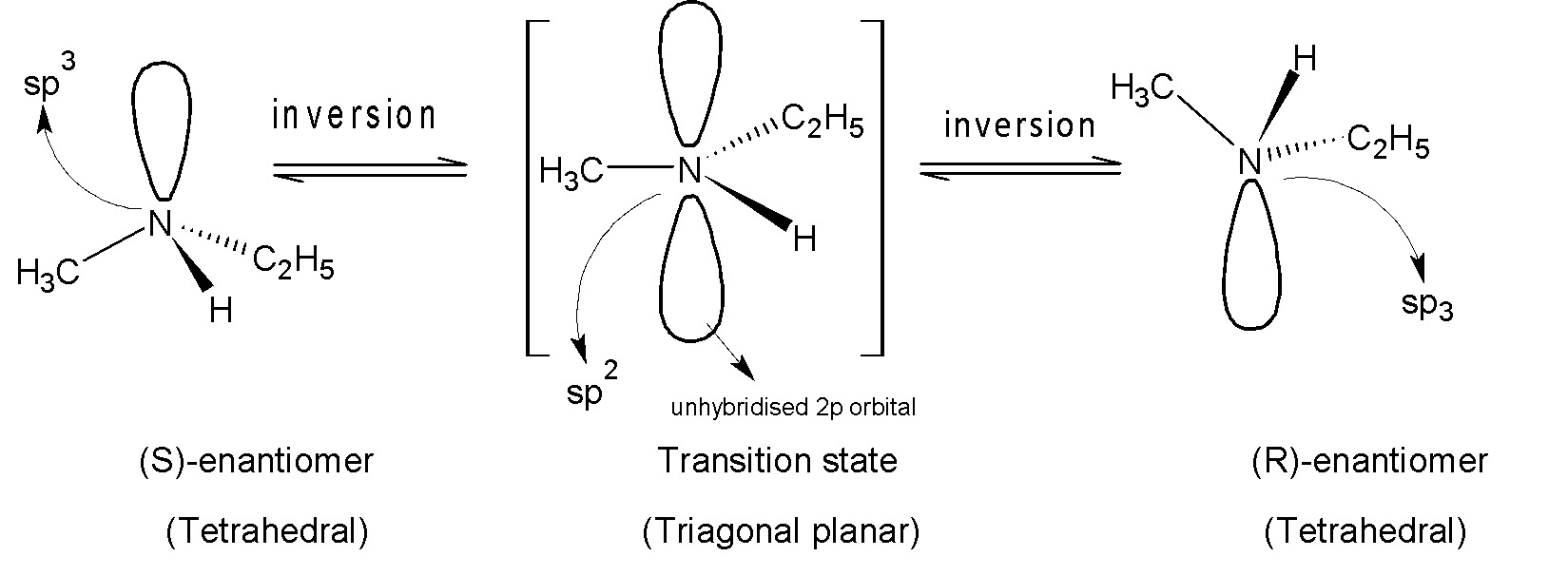
So, chiral ethyl methyl amine can not be resolved due to its pyramidal inversion and very rapidly inverts its configuration to a mixture. Options (B)and (D) are not the reason for resolving chiral amines.
Thus, option (A) is correct.
Note: Due to the presence of lone pair of electrons secondary and tertiary amine faces a repulsion between bond pair and lone pair. Consequently, the bond angle between two alkyl groups decreases from ${{109.7}^{{\mathrm O}}}$to ${{107}^{{\mathrm O}}}$. To reduce this repulsion amines undergo rapid inversion to trigonal planar with bond angle ${{120}^{{\mathrm O}}}$.
Complete step by step solution:
Secondary and tertiary amines with three different groups bonded to nitrogen are generally chiral compounds that can not be easily resolved at room temperature. Because nitrogen inversion or pyramidal inversion rapidly inverts the two enantiomers.
We have ethyl methylamine which is a chiral compound as the nitrogen center has four different groups (-Et, -Me, –H group and a lone pair of electrons) in other words it has non-superimposable mirror images. The structure of chiral ethyl methylamine is shown below:

Nitrogen atom bonded with three groups possesses $s{{p}^{3}}$ hybridization and trigonal pyramidal geometry. At room temperature, the two arrangements of ethyl methylamine are in equilibrium and rapidly invert its stereochemistry via trigonal planar,$s{{p}^{2}}$ nitrogen atom where the lone pair of electrons lie in the unhybridized $2p$ orbital. During this inversion, there is a rapid oscillation of nitrogen atoms from one side of the plane to another side of the plane. Like this nitrogen completes its inversion process and back to its $s{{p}^{3}}$ hybridization.

So, chiral ethyl methyl amine can not be resolved due to its pyramidal inversion and very rapidly inverts its configuration to a mixture. Options (B)and (D) are not the reason for resolving chiral amines.
Thus, option (A) is correct.
Note: Due to the presence of lone pair of electrons secondary and tertiary amine faces a repulsion between bond pair and lone pair. Consequently, the bond angle between two alkyl groups decreases from ${{109.7}^{{\mathrm O}}}$to ${{107}^{{\mathrm O}}}$. To reduce this repulsion amines undergo rapid inversion to trigonal planar with bond angle ${{120}^{{\mathrm O}}}$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




