
What is the electronic configuration for a gas phase +3 ion of iron (Z = 26)?
(A) $\left[ Ar \right]3{{d}^{5}}$
(B) $\left[ Ar \right]4{{s}^{2}}3{{d}^{3}}$
(C) $\left[ Ar \right]4{{s}^{1}}3{{d}^{4}}$
(D) $\left[ Ar \right]4{{s}^{2}}3{{d}^{6}}$
Answer
233.1k+ views
Hint: Use the atomic number of iron given to write the electronic configuration of iron. A positive charge means electrons are deficient and a negative charge means electrons are in excess. Here it has a +3 positive charge therefore there we have to consider 3 fewer electrons while writing the configuration.
Complete step by step solution:
Electronic configuration of an atom or molecule gives us the numeric arrangement of electrons around the nucleus.
There are specific notations that we use for writing the configuration of an atom. For writing these notations, we start with the energy orbitals. Practically, there are 4 orbitals s, p, d and f. There is a certain even number of the electrons that each orbital can accommodate. s-orbital can accommodate 2 electrons whereas p, d and f-orbitals can accommodate 6, 10 and 14 electrons respectively.
There is a trend each electron follows while filling these orbitals and it is given as-
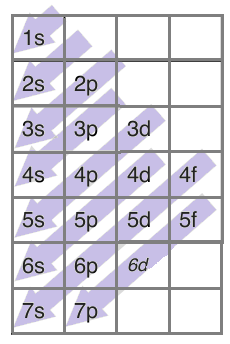
Following this trend, we can write the electronic configuration of the +3 state of iron.
We know that the atomic number of iron is 26. In +3 state it will lose 3 electrons therefore, we will be left with 23electrons. Following the above trend, we can write the electronic configuration of $F{{e}^{3+}}$ as- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}$
We know that the atomic number of argon is 18. Therefore, electronic configuration of argon is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$ .
As we know argon lies in the third period in the periodic table and iron lies in the fourth period. Therefore, we can write the electronic configuration of iron in terms of the argon as- $\left[ Ar \right]3{{d}^{5}}$ .
Therefore, the correct answer is option (A) $\left[ Ar \right]3{{d}^{5}}$.
Note: According to Pauli’s Exclusion Principle, each orbital can hold 2 electrons. s-orbital set contains one orbital, thus can hold 2 electrons. Similarly, the p-orbital set had three orbitals, thus can hold 6 electrons and d-orbital and f-orbital have five and seven orbitals thus, can hold 10 and 14 electrons respectively.
Complete step by step solution:
Electronic configuration of an atom or molecule gives us the numeric arrangement of electrons around the nucleus.
There are specific notations that we use for writing the configuration of an atom. For writing these notations, we start with the energy orbitals. Practically, there are 4 orbitals s, p, d and f. There is a certain even number of the electrons that each orbital can accommodate. s-orbital can accommodate 2 electrons whereas p, d and f-orbitals can accommodate 6, 10 and 14 electrons respectively.
There is a trend each electron follows while filling these orbitals and it is given as-

Following this trend, we can write the electronic configuration of the +3 state of iron.
We know that the atomic number of iron is 26. In +3 state it will lose 3 electrons therefore, we will be left with 23electrons. Following the above trend, we can write the electronic configuration of $F{{e}^{3+}}$ as- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}$
We know that the atomic number of argon is 18. Therefore, electronic configuration of argon is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$ .
As we know argon lies in the third period in the periodic table and iron lies in the fourth period. Therefore, we can write the electronic configuration of iron in terms of the argon as- $\left[ Ar \right]3{{d}^{5}}$ .
Therefore, the correct answer is option (A) $\left[ Ar \right]3{{d}^{5}}$.
Note: According to Pauli’s Exclusion Principle, each orbital can hold 2 electrons. s-orbital set contains one orbital, thus can hold 2 electrons. Similarly, the p-orbital set had three orbitals, thus can hold 6 electrons and d-orbital and f-orbital have five and seven orbitals thus, can hold 10 and 14 electrons respectively.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




