
Compound that is both paramagnetic and coloured is:
A) $\text{ }{{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{ }$
B) $\text{ (N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{ }\!\![\!\!\text{ TiC}{{\text{l}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$
C) $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$
D) $\,\text{ }{{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Cu(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$
Answer
241.2k+ views
Hint: The central metal ion in the complex makes available a number of empty orbitals for the formation of coordination bonds with suitable ligands. The central metal ion excites and loses its d shell electrons. After excitation, if d-orbitals have unpaired electrons then it is said to be paramagnetic otherwise diamagnetic. The colour of the complex is due to the excitation of an electron from a metal ion on the absorption of visible radiation. The colour of the complex is complementary to the colour absorbed by the metal.
Complete step by step solution:
Let's have a look at the coordination compound of vanadium with sulphate. The complex is given as, $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$. The central vanadium metal atom has the electronic configuration as,
$\text{ V = }\left[ Ar \right]\text{ 3}{{\text{d}}^{3}}\text{ 4}{{\text{s}}^{\text{2}}}\text{ }$
Here, in$\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$, the vanadium is in $\text{ + 3 }$ an oxidation state thus electronic configuration on excitation is,
$\text{ V = }\left[ Ar \right]\text{ 3}{{\text{d}}^{2}}\text{ 4}{{\text{s}}^{0}}\text{ }$
When the sulphate ion approaches towards the metal ion, the electrons of the ligand do not disturb the sulphate ion. The sulphate ions donate its two electrons and oxide donate one electron. The d orbitals are as show below,
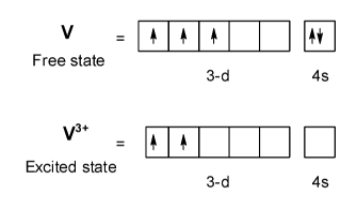
From the above representation, it is clear that the two 3d orbitals of the vanadium acquire an unpaired electron in it. Thus, $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ is paramagnetic. The vanadium is present as the vanadyl ion $\text{ V}{{\text{O}}^{\text{2+}}}\text{ }$. The complex is usually written as $\text{ }{{\left[ \text{V}{{\text{O}}_{\text{2}}}{{\text{(}{{\text{H}}_{\text{2}}}\text{O)}}_{\text{4}}} \right]}^{\text{2+}}}\text{ }$. This hydrated complex is responsible for the colour of the complex.
The $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ generally exist as the hydrated complex $\text{ }{{\left[ \text{V}{{\text{O}}_{\text{2}}}{{\text{(}{{\text{H}}_{\text{2}}}\text{O)}}_{\text{4}}} \right]}^{\text{2+}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$. It is obtained as the vanadium (III) sulphate tetrahydrate. It exists as a blue crystalline solid. The colour of the complex can be explained based on the transition of electrons from the lower energy level to the higher energy level. The two unpaired electrons of vanadium absorb the radiation and undergo excitation.
The transition metals, vanadium absorbs the radiation from the visible region and transmits the radiation. The colour we see is due to the light which is not absorbed but which is transmitted called a complementary colour. $\text{ }{{\text{V}}^{\text{3+}}}\text{ }$ When exposed to visible radiation absorb the orange-red radiations thus $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ is blue.
$\text{ }{{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{ }$ is a diamagnetic and colourless compound.
The $\text{ (N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{ }\!\![\!\!\text{ TiC}{{\text{l}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$ is a diamagnetic and colourless complex.
In $\,\text{ }{{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Cu(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$ , copper exists in $\text{ +1 }$ an oxidation state. Cu (I) compounds are usually colourless or white. Thus, it is diamagnetic.
Thus, $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ is both paramagnetic and colour.
Hence, (C) is the correct option.
Note: It may be noted that only those transition metal complexes are coloured which have incompleted-subshells.The transition metal ions having filled or empty d-subshells are colourless. For example, complexes of $\text{ C}{{\text{u}}^{\text{+}}}\text{ (}{{\text{d}}^{\text{10}}}\text{) }$ , $\text{ Z}{{\text{n}}^{\text{2+}}}({{d}^{10}})$ , $\text{ A}{{\text{g}}^{\text{+}}}\text{ (}{{\text{d}}^{\text{10}}}\text{) }$ , etc. The colour is due to the d-d transition of electrons.
Complete step by step solution:
Let's have a look at the coordination compound of vanadium with sulphate. The complex is given as, $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$. The central vanadium metal atom has the electronic configuration as,
$\text{ V = }\left[ Ar \right]\text{ 3}{{\text{d}}^{3}}\text{ 4}{{\text{s}}^{\text{2}}}\text{ }$
Here, in$\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$, the vanadium is in $\text{ + 3 }$ an oxidation state thus electronic configuration on excitation is,
$\text{ V = }\left[ Ar \right]\text{ 3}{{\text{d}}^{2}}\text{ 4}{{\text{s}}^{0}}\text{ }$
When the sulphate ion approaches towards the metal ion, the electrons of the ligand do not disturb the sulphate ion. The sulphate ions donate its two electrons and oxide donate one electron. The d orbitals are as show below,

From the above representation, it is clear that the two 3d orbitals of the vanadium acquire an unpaired electron in it. Thus, $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ is paramagnetic. The vanadium is present as the vanadyl ion $\text{ V}{{\text{O}}^{\text{2+}}}\text{ }$. The complex is usually written as $\text{ }{{\left[ \text{V}{{\text{O}}_{\text{2}}}{{\text{(}{{\text{H}}_{\text{2}}}\text{O)}}_{\text{4}}} \right]}^{\text{2+}}}\text{ }$. This hydrated complex is responsible for the colour of the complex.
The $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ generally exist as the hydrated complex $\text{ }{{\left[ \text{V}{{\text{O}}_{\text{2}}}{{\text{(}{{\text{H}}_{\text{2}}}\text{O)}}_{\text{4}}} \right]}^{\text{2+}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$. It is obtained as the vanadium (III) sulphate tetrahydrate. It exists as a blue crystalline solid. The colour of the complex can be explained based on the transition of electrons from the lower energy level to the higher energy level. The two unpaired electrons of vanadium absorb the radiation and undergo excitation.
The transition metals, vanadium absorbs the radiation from the visible region and transmits the radiation. The colour we see is due to the light which is not absorbed but which is transmitted called a complementary colour. $\text{ }{{\text{V}}^{\text{3+}}}\text{ }$ When exposed to visible radiation absorb the orange-red radiations thus $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ is blue.
$\text{ }{{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{ }$ is a diamagnetic and colourless compound.
The $\text{ (N}{{\text{H}}_{\text{4}}}{{\text{)}}_{\text{2}}}\text{ }\!\![\!\!\text{ TiC}{{\text{l}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }$ is a diamagnetic and colourless complex.
In $\,\text{ }{{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Cu(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }$ , copper exists in $\text{ +1 }$ an oxidation state. Cu (I) compounds are usually colourless or white. Thus, it is diamagnetic.
Thus, $\text{ VO(S}{{\text{O}}_{\text{4}}}\text{)}$ is both paramagnetic and colour.
Hence, (C) is the correct option.
Note: It may be noted that only those transition metal complexes are coloured which have incompleted-subshells.The transition metal ions having filled or empty d-subshells are colourless. For example, complexes of $\text{ C}{{\text{u}}^{\text{+}}}\text{ (}{{\text{d}}^{\text{10}}}\text{) }$ , $\text{ Z}{{\text{n}}^{\text{2+}}}({{d}^{10}})$ , $\text{ A}{{\text{g}}^{\text{+}}}\text{ (}{{\text{d}}^{\text{10}}}\text{) }$ , etc. The colour is due to the d-d transition of electrons.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




