
\[C{{l}_{2}}\] reacts with \[C{{S}_{2}}\] in presence of \[{{I}_{2}}\] catalyst to form
(A) \[CHC{{l}_{3}}\]
(B) \[CC{{l}_{4}}\]
(C) \[{{C}_{2}}{{H}_{5}}Cl\]
(D) \[{{C}_{2}}{{H}_{6}}\]
Answer
233.1k+ views
Hint: Interaction between polar and nonpolar molecules is due to London dispersion force or induced dipole. In this type of interaction, at any particular time, both molecules acquire a temporary dipole.
Complete Step by Step Answer:
A bond between two chlorine atoms to form chlorine is a covalent bond as both chlorine atoms are non-metal. Now covalent bonds are of two types, one is polar, and the other is non-polar. Chlorine is non-polar as there is no electronegativity difference between two chlorine atoms.
Also, carbon disulfide is non-polar as both are non-metal however, there is a small electronegativity difference between carbon and sulphur but sulphur bonded with carbon with two double bonds and thus, both sides dipole cancel out and the molecule becomes non-polar.
When \[C{{l}_{2}}\] reacts with \[C{{S}_{2}}\]in the presence of \[{{I}_{2}}\]catalyst then the negative temporary positive pole of chlorine molecule will interact with the temporary negative pole of carbon disulfide and the negative temporary pole of chlorine molecule will interact with the positive temporary pole of carbon disulfide in the presence of iodine catalyst to give carbon tetrachloride such as
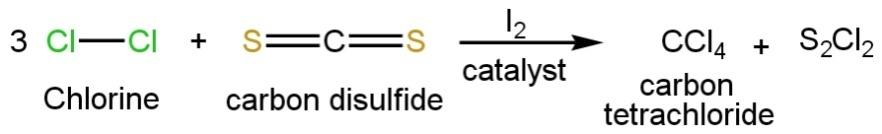
Thus, the correct option is B.
Note: In this reaction, iodine catalyst has been used as iodine is majorly used in the process of conversion of unstable reaction or compound to a stable reaction or stable compound. Carbon tetrachloride is more stable than carbon disulfide. Carbon tetrachloride can also be prepared when chlorine reacts with methane.
Complete Step by Step Answer:
A bond between two chlorine atoms to form chlorine is a covalent bond as both chlorine atoms are non-metal. Now covalent bonds are of two types, one is polar, and the other is non-polar. Chlorine is non-polar as there is no electronegativity difference between two chlorine atoms.
Also, carbon disulfide is non-polar as both are non-metal however, there is a small electronegativity difference between carbon and sulphur but sulphur bonded with carbon with two double bonds and thus, both sides dipole cancel out and the molecule becomes non-polar.
When \[C{{l}_{2}}\] reacts with \[C{{S}_{2}}\]in the presence of \[{{I}_{2}}\]catalyst then the negative temporary positive pole of chlorine molecule will interact with the temporary negative pole of carbon disulfide and the negative temporary pole of chlorine molecule will interact with the positive temporary pole of carbon disulfide in the presence of iodine catalyst to give carbon tetrachloride such as

Thus, the correct option is B.
Note: In this reaction, iodine catalyst has been used as iodine is majorly used in the process of conversion of unstable reaction or compound to a stable reaction or stable compound. Carbon tetrachloride is more stable than carbon disulfide. Carbon tetrachloride can also be prepared when chlorine reacts with methane.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




