
Chlorobenzene can be prepared by reacting aniline with:
(A) Hydrochloric acid (HCl)
(B) Cuprous chloride $\left( C{{u}_{2}}C{{l}_{2}} \right)$
(C) Chlorine in the presence of anhydrous aluminium chloride
(D) Nitrous acid followed by heating with cuprous chloride (CuCl)
Answer
232.8k+ views
Hint: Start by drawing the structure of the reactant. Think what kind of reactions aniline undergoes with the given reagents in the options. The product formed should not contain any other substituent on the ring except chlorine.
Complete step by step solution:
-Aniline is an aromatic amine. It is a base.
-Let’s have a look at the question. We need to prepare chlorobenzene from aniline. That means the amine group needs to be replaced by a chlorine group.
-We have studies that amine group can only be replaced after it has been converted to diazonium salt form, which is unstable. Direct halogenation of aniline will give rise to halogen derivatives of aniline.
-If HCl is added to aniline, then aniline being a base will abstract the proton and form anilinium chloride salt.
-When aniline is treated with nitrous acid, which is produced in situ by sodium nitrite and hydrochloric acid, at low temperature $\left( 0{}^\circ C-5{}^\circ C \right)$, then aniline is converted to a benzene diazonium salt. This reaction is known as diazotization.
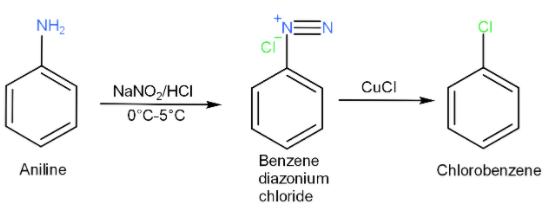
-When a freshly prepared solution of benzene diazonium salt is treated with cuprous chloride (CuCl), it gives chlorobenzene. This reaction is known as Sandmeyer’s reaction.
-Therefore, chlorobenzene can be prepared by reacting aniline with nitrous acid followed by heating with cuprous chloride (CuCl).
Therefore, the correct option is (D).
Note: Chlorobenzene can also be prepared by treating benzene diazonium salt with copper powder and hydrochloric acid. This reaction is known as Gattermann reaction and has a better yield than Sandmeyer reaction. Remember in aromatic compounds electrophilic substitution reactions occur readily and therefore, direct halogenation will yield halo-substituted compounds.
Complete step by step solution:
-Aniline is an aromatic amine. It is a base.
-Let’s have a look at the question. We need to prepare chlorobenzene from aniline. That means the amine group needs to be replaced by a chlorine group.
-We have studies that amine group can only be replaced after it has been converted to diazonium salt form, which is unstable. Direct halogenation of aniline will give rise to halogen derivatives of aniline.
-If HCl is added to aniline, then aniline being a base will abstract the proton and form anilinium chloride salt.
-When aniline is treated with nitrous acid, which is produced in situ by sodium nitrite and hydrochloric acid, at low temperature $\left( 0{}^\circ C-5{}^\circ C \right)$, then aniline is converted to a benzene diazonium salt. This reaction is known as diazotization.

-When a freshly prepared solution of benzene diazonium salt is treated with cuprous chloride (CuCl), it gives chlorobenzene. This reaction is known as Sandmeyer’s reaction.
-Therefore, chlorobenzene can be prepared by reacting aniline with nitrous acid followed by heating with cuprous chloride (CuCl).
Therefore, the correct option is (D).
Note: Chlorobenzene can also be prepared by treating benzene diazonium salt with copper powder and hydrochloric acid. This reaction is known as Gattermann reaction and has a better yield than Sandmeyer reaction. Remember in aromatic compounds electrophilic substitution reactions occur readily and therefore, direct halogenation will yield halo-substituted compounds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




