




What are Covalent Bonds?
A chemical bond, known as a covalent bond, involves the sharing of electron pairs between atoms. When atoms share electrons, a stable equilibrium of the attractive and repulsive forces between them is known as covalent bonding. These electron pairs are also known as shared pairs or bonding pairs. A covalent bond is a bond that binds two atoms together because the word co-denotes ‘jointly and connected in action’. The two atoms just need to have comparable electronegativity in order to form a covalent connection; they need not be from the same element.
Delocalised covalent bonding is defined as covalent bonding in which electrons are shared by more than two atoms. A covalent bond is always formed by the overlapping of orbitals. A bond brings stability to a molecule, accompanied by the release of energy. An example of a covalent bond.
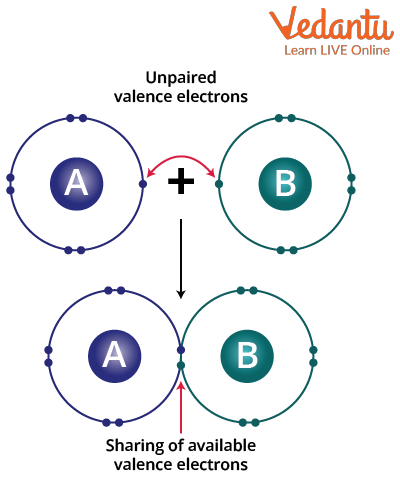
Covalent Bond
Characteristics of Covalent Bonds
A covalent bond is formed by the sharing of electrons due to which the force of attraction between the molecules is less as compared to ionic compounds. Ionic bonds are formed by the loss or gain of electrons. Covalent compounds are thus found in a gaseous or liquid state.
Covalent compounds generally have low melting and boiling points. Covalent bonds are directional in nature. Covalent bonds do not generally conduct electricity; however, if electron conjugation is present in a compound, it does. Covalent bonds are insoluble in water and require an energy of approximately 80 kcal to break a covalent bond. A covalent bond is formed in order to complete an octet.
Covalent Bonding in Carbon
Carbon has an atomic weight of 6, and its electronic structure is 2,4. As a result, the outermost shell of carbon has 4 valence electrons. The electrons in an atom's outermost energy level are known as valence electrons that play a role in the formation of chemical bonds. As a result, covalent bonds can be created between carbon atoms or between carbon has a tetravalent nature, meaning that it can make 4 chemical bonds to complete its octet by sharing electrons. Carbon atoms and atoms from other elements. Hydrogen and carbon frequently form bonds. Hydrocarbons are compounds that only contain carbon and hydrogen. For example, ethane C2H6.
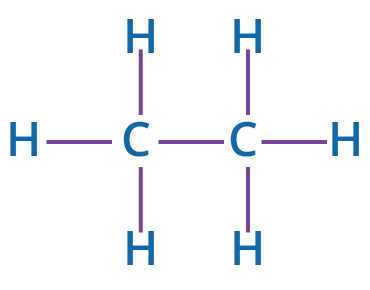
Ethane Molecule
A carbon atom can form covalent bonds with itself and other atoms, such as oxygen, sulphur, nitrogen, and hydrogen, among others, leading to the possibility of a large number of molecules. This is because carbon is tetravalent, has a high bond energy, and has a small atomic size. As a result, it exhibits the highest degree of catenation.
Bonding in Allotropes of Carbon
Allotropes are one or more chemical elements' physical forms that can exist in the same physical state. The chemical and physical characteristics of allotropes may differ. The same forces—light, pressure, and temperature—that affect other structures also affect allotropic forms. Due to the catenation property of carbon, there exist many allotropes of carbon. The important allotropes of carbon that exist in nature are diamond and graphite.
Bonding in the Diamond
A face-centred cubic lattice with eight atoms per unit cell makes up the diamond's crystal structure, which has a cubic shape. In a tetrahedral structure, each carbon atom is covalently connected to four other carbon atoms. Diamond has a 3-dimensional network of six-membered carbon rings. A C-C bond length of 154 pm results from the bonding through sp3 hybridised orbitals. Diamond is incredibly strong due to its network of flexible covalent connections. At low pressures, diamond is thermodynamically less stable than graphite.
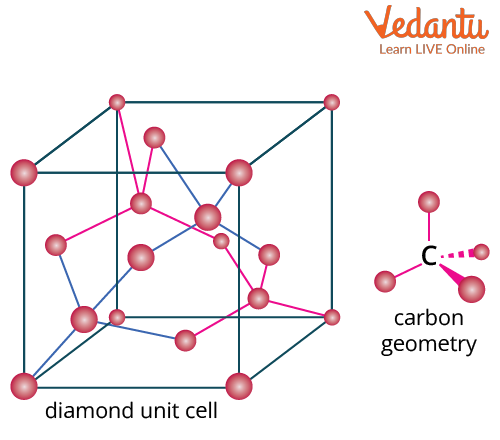
Bonding in Diamond
Bonding in Graphite
Due to the delocalisation of the pi bond electrons above and below the planes of the carbon atoms, graphite conducts electricity. To create simple bonds with its three nearest neighbours, each carbon atom requires three of its electrons. So, the bonding level is left with one extra electron. Each carbon atom's "spare" electrons spread out across the entire sheet of atoms in a layer.
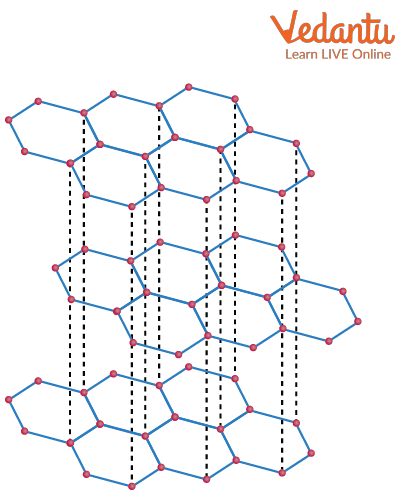
Bonding in Graphite
The fact that the delocalised electrons are no longer tied to specific carbon atoms and are instead free to roam anywhere in the sheet is important. The delocalised electrons in one sheet do not, however, directly interact with those in the adjacent sheets. Strong covalent bonds hold the atoms in a sheet together; these bonds are stronger than those in diamond due to the extra bonding that the delocalised electrons produce.
Conclusion
A chemical bond known as a covalent bond involves the sharing of electron pairs between atoms. When atoms share electrons, a stable equilibrium of the attractive and repulsive forces between them is known as covalent bonding. We also looked at a covalent bond example in the context.
Carbon has a tetravalent nature, meaning that it can make four chemical bonds to complete its octet by sharing electrons. Carbon bonding in particular is of great significance in organic chemistry as the whole branch is based on it. Due to the catenation property of carbon, there exist many allotropes of carbon. The important allotropes of carbon that exist in nature are diamond and graphite.
FAQs on Covalent Bonding in Carbon for JEE
1. How many types of covalent bonds are there?
There are three types of covalent bonds:
- Single bonds
- Double bonds
- Triple bonds.
When just one pair of electrons is shared by the two participating atoms, a single bond is created. It is denoted by a single dash. It is the kind of bond that is the most stable. A double bond is created when two pairs of electrons are shared by the two participating atoms. It is stronger than single bonds but less stable. A triple bond is created when three pairs of electrons are shared by the two participating atoms. These bonds are the least stable but are very strong.
2. Explain bonding in fullerenes.
Carbon atoms form hollow molecules called fullerenes. Their structures are based on covalently linked hexagonal rings of carbon atoms. Rings of five or six carbon atoms can be found in several fullerenes. Buckminsterfullerene and nanotubes are two examples of fullerenes. Buckminsterfullerene molecules are held together by weak intermolecular forces. Buckminsterfullerene is smooth and has a low melting point. Therefore, it requires little energy to overcome the forces between them.
A nanotube resembles a layer of graphene that has been wrapped up into a tube. Nanotubes have high length to diameter ratios because their length is exceptionally long relative to their width.
























