




Introduction to Addition or Molecular Compounds
Two or more simpler compounds that can fit into a crystal in a specific ratio make up an addition compound. The phrase refers to a number of lattice compounds as well as donor-acceptor complexes (adducts).
Chemical substances known as molecular compounds assume the shape of distinct molecules. Examples include common compounds like carbon dioxide(CO2) and water (H2O).
In this article, we will discuss briefly about the addition and molecular compounds, their types and examples along with the properties they possess. Lastly, we will discuss the differences between compounds and a molecule, which is important to be known.
Addition Compounds
Addition compounds are created when two or more stable compounds are mixed together in stoichiometric amounts.
$\begin{align} &\mathrm{K}_{2} \mathrm{SO}_{4}+\mathrm{Al_2}\left(\mathrm{SO}_{4}\right)_{3}+24 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{K}_{2} \mathrm{SO}_{4} \cdot \mathrm{Al}_{2}\left(\mathrm{SO}_{4}\right)_{3} \cdot 24 \mathrm{H}_{2} \mathrm{O}(\text { Potash alum) } \\ &\mathrm{CuSO}_{4}+4 \mathrm{NH}_{3} \rightarrow \mathrm{CuSO}_{4} \cdot 4 \mathrm{NH}_{3} \text { ( Tetraammine copper sulfate) } \end{align}$
These Addition Compounds Come in Two Varieties:
(I) Double Salts or Lattice Compounds: These addition compounds break into its component parts when dissolved in water or melted, yet stay stable in the solid state.
When dissolved in water, these salts completely dissociate to give all of the ions, received by the compounds with which they are formed.
The molar conductivity of such a solution corresponds to all of the ions provided by the constituent compounds.
For instance, the K+, Al+3, and SO4-2 ions characteristics can be seen in an aqueous solution of potash alum.
II) Coordination Compounds: These addition compounds retain their identity in both the solid and dissolved states, but typically lose the unique characteristics of their constituents.
In solution, these compounds do not provide all of the ions provided by the constituent compounds.
In the example blue tetraamine copper sulphate solution, cupro ammonium ions $[Cu(NH_3)_4]^{+2}$ are present rather than Cu+2 ions, which would indicate the existence of Cu+2 ions. These substances are made up of a core metal atom or ion, surrounded by the right number of ions or neutral molecules.
Molecular Compounds
A molecular compound is made up of molecules, whose chemical composition reflects the amount of atoms that are really bound together in the molecule. The bond lengths and angles between the atoms bonds together to give the compound a distinct form.
Below are a few illustrations:
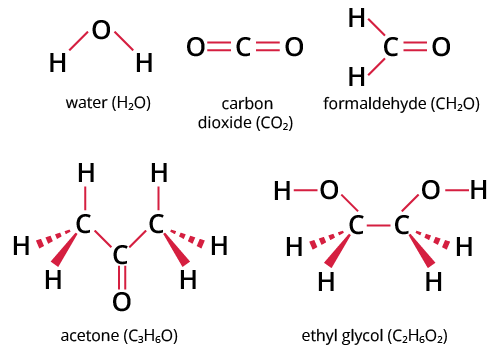
Examples of Molecular Compounds
Typically, two or more non-metal components make up a molecular complex. The first element is named first, followed by the second element, using the stem of the element name plus the suffix. The number of atoms in a molecule is specified using numerical prefixes.
Chemical substances known as molecular compounds assume the shape of distinct molecules. Examples include common substances like carbon dioxide and water (H2O) (CO2).
Properties of Molecular compounds
The following are the characteristics of molecular compounds:
They have much lower melting and boiling temperatures. This is due to how little energy is needed to overcome the intermolecular interactions that hold molecules together.
The molecular compounds' weak electrical conductivity results from the fact that they are made up of neutral molecules.
Molecular compounds' water solubility varies and is mostly influenced by the nature of the intermolecular interactions at play.
Covalent compounds rarely exhibit well-organised 3-dimensional structures due to the relatively weak partial charges.
Molecules of Compounds Examples
Compound molecules contain atoms from two or more distinct elements. As an illustration, the molecule of water (H2O) contains three atoms: two hydrogen (H) atoms and one oxygen (O) atom.
Common greenhouse gas methane (CH4) has five atoms: one carbon (C) and four hydrogen atoms (H).
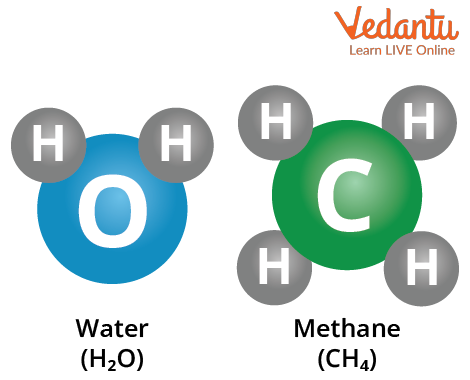
Examples of Molecular Compounds.
Difference between Molecule and Compound with Examples
Some molecules can be compounds such as H2O, HNO3.
Conclusion
From this article, we come to a conclusion that, when a solution is made by mixing two or more simple stable compounds in simple molecular proportions and is allowed to evaporate, crystals of a new substance may separate out, which are called addition compounds. Examples of these compounds are already discussed.
A molecular compound is composed of molecules whose chemical composition reflects the number of atoms actually bound together in the molecule. They also possess many different properties. Their common examples are molecules of water (H2O), methane (CH4) etc.
We have also discussed the differences between the molecule and compound which helped us to deal with the topic in a better way.
FAQs on Addition or Molecular Compounds and Their Examples for JEE
1. Difference between ionic and molecular compounds?
The differences between ionic and molecular compounds are discussed below:
Molecular compounds are pure substances formed when atoms are linked together by electron sharing, whereas ionic compounds are formed by electron transfer.
Molecular compounds are formed through covalent bonding, whereas ionic compounds are formed through ionic bonding.
Generally, molecular compounds are formed when two non-metals react, whereas ionic compounds are formed when metals and non-metals react.
Molecular compounds conduct electricity poorly, whereas ionic compounds conduct electricity well.
Molecular compounds can exist in any physical state, including solid, liquid and gas. Ionic compounds mostly appear solid and crystalline.
More molecular compounds exist than ionic compounds.
2. What are compounds? Give some examples.
A compound is a substance made up of two or more chemical elements that have their atoms bonded together. These atoms are chemically bonded in precise ways and proportions, and the substances cannot be easily separated using simple physical means.
Compounds come in a variety of forms, including binary, ionic, molecular, acids, cations and anions. These compounds have various properties and chemical compositions.
Examples of compounds are:
Water(H2O) = Hydrogen2 + Oxygen
Hydrogen Peroxide(H2O2) = Hydrogen2 + Oxygen2
Sodium Chloride (NaCl) = Sodium + Chlorine
Baking Soda (NaHCO3) = Sodium + Hydrogen + Carbon + Oxygen3
Octane (C8H18) = Carbon8 + Hydrogen18 .

































