
By increasing the temperature, the specific resistance of a conductor _________ and that of a semiconductor _________.
A) increases for both
B) decreases for both
C) increases, decreases
D) decreases, increases
Answer
232.8k+ views
Hint: The materials are classified into three different categories based on electrical conductivity as: Conductors, Semi-conductors and Insulators. While the conductors are good conductors of electricity and insulators are bad conductors of electricity, the conductivity of semiconductors is better than that of insulators but not as good as the conductors.
Complete answer:
Based on the electrical conductivity, we have three categories based on the relative order of electrical conductivity as:
Insulators < Semiconductors < Conductors.
The main criteria that determines the conductivity in the materials is the energy gap between the conduction and valence band.
The valence band consists of the electrons in the outermost shell of the atom and the conduction band consists of electrons that conduct electricity.
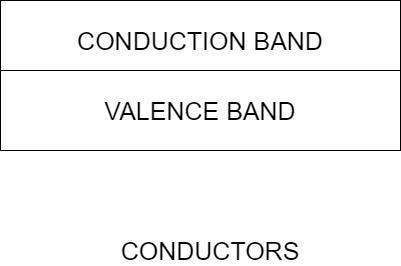
For conductors, the valence and the conduction band overlap. This means that the valence electrons in the conductors are readily available for conduction.
For insulators, the valence band and conduction band are very distant and the energy gap is too high. This means that the electrons in the valence band can never reach the conduction band for conducting.

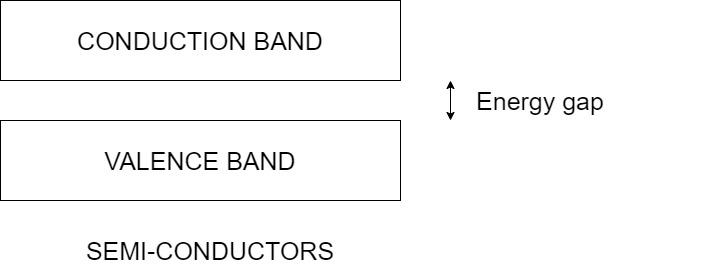
However, for semiconductors, the valence band and conduction are at a small distance and not too large for the valence electrons to overcome. Hence, the electrons in the valence can be excited to the conduction band to help in conduction.
The resistivity or specific resistance of the material is the innate property of a material by virtue of which the flow of electrons is opposed when it is subject to an external potential difference.
It is represented by $\rho $ and measured in the units $\Omega - m$.
In semiconductors, when the electrons are energised to the conduction band by increasing the temperature, they start conducting and hence, their resistivity decreases.
So, in semiconductors, with the increase in temperature, the resistivity decreases.
In the case of conductors, the velocity of the electrons in the conduction band increases. But, this also leads to an increase in vibration of the metallic atoms in the interior which effectively, offers hindrance or obstruction to the velocity of the free electrons, which decreases its conductivity.
Thus, the resistivity increases with temperature in the case of conductors.
Hence, by increasing the temperature, the specific resistance of a conductor increases and that of a semiconductor decreases.
Hence, the correct option is Option C.
Note: Increasing temperature is not the only way to aid the conduction in semiconductors. This is only the case for pure semiconductors. To make a semiconductor operational at room temperature, the semiconductor is mixed with another element known as dopant making it an extrinsic semiconductor. The extrinsic semiconductor is more common than the pure semiconductor.
Complete answer:
Based on the electrical conductivity, we have three categories based on the relative order of electrical conductivity as:
Insulators < Semiconductors < Conductors.
The main criteria that determines the conductivity in the materials is the energy gap between the conduction and valence band.
The valence band consists of the electrons in the outermost shell of the atom and the conduction band consists of electrons that conduct electricity.
For conductors, the valence and the conduction band overlap. This means that the valence electrons in the conductors are readily available for conduction.

For insulators, the valence band and conduction band are very distant and the energy gap is too high. This means that the electrons in the valence band can never reach the conduction band for conducting.

However, for semiconductors, the valence band and conduction are at a small distance and not too large for the valence electrons to overcome. Hence, the electrons in the valence can be excited to the conduction band to help in conduction.

The resistivity or specific resistance of the material is the innate property of a material by virtue of which the flow of electrons is opposed when it is subject to an external potential difference.
It is represented by $\rho $ and measured in the units $\Omega - m$.
In semiconductors, when the electrons are energised to the conduction band by increasing the temperature, they start conducting and hence, their resistivity decreases.
So, in semiconductors, with the increase in temperature, the resistivity decreases.
In the case of conductors, the velocity of the electrons in the conduction band increases. But, this also leads to an increase in vibration of the metallic atoms in the interior which effectively, offers hindrance or obstruction to the velocity of the free electrons, which decreases its conductivity.
Thus, the resistivity increases with temperature in the case of conductors.
Hence, by increasing the temperature, the specific resistance of a conductor increases and that of a semiconductor decreases.
Hence, the correct option is Option C.
Note: Increasing temperature is not the only way to aid the conduction in semiconductors. This is only the case for pure semiconductors. To make a semiconductor operational at room temperature, the semiconductor is mixed with another element known as dopant making it an extrinsic semiconductor. The extrinsic semiconductor is more common than the pure semiconductor.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding Uniform Acceleration in Physics

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students




