
Assume that the nuclear binding energy per nucleus (B/A) versus mass number (A) as shown in the figure. Use this plot to choose the correct choice given below
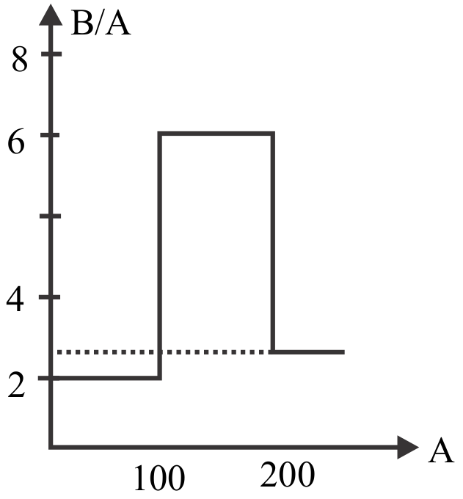
(A) Fusion of two nuclei with mass number lying in the range of $50 < A < 100$ will release energy
(B) Fission of a nucleus lying in the mass range $200 < A < 260$ will release energy when (C) broken into two equal fragments
(C) Both (A) and (B)
(D) None of the above
Answer
240.3k+ views
Hint: In the given bar graph, mass number is plotted against the binding energy per nucleus. We have to analyse the given options and we have to find the correct statement regarding the energy released due to fission and fusion process.
Complete step by step answer:
When two lighter nuclei combine to form a stable nucleus, some mass of proton and neutron disappears, similarly when a nucleus get split into two lighter nuclei, here also some mass of proton and neutron get disappeared, the mass that disappears is converted into an equivalent amount of energy which is known as the binding energy of the nucleus
Nuclear fusion is the process of combining two or more nuclei to form a heavy nucleus. During this process a huge amount of energy is released.
Nuclear fission is the process of splitting an unstable nucleus to get two stable nuclei. During this process a huge amount of energy is released.
From the given graph we can see that the nucleus with mass number from \[100{\text{ }}to{\text{ }}200\] has a large binding energy.
So, if two lighter nuclei combine to form a stable nucleus of mass number between \[100{\text{ }}and{\text{ }}200\] then it will release large energy since it has large binding energy.
And also if the heavy nucleus splits into two lighter nuclei having mass number between \[100{\text{ }}and{\text{ }}200\] then it will release large energy since it has large binding energy.
Which means the nucleus formed by fusion should have mass number between \[100{\text{ }}and{\text{ }}200\] and the lighter nuclei formed by fission process should have mass number between \[100{\text{ }}and{\text{ }}200\] to release large amount of energy.
To form a nucleus having mass number between 100 and 200 we should use the lighter nuclei of mass number between 51 and 100. So that it will combine and the mass numbers of the two nuclei will get added and the resultant mass number will lie between 100 and 200.
To get two lighter nuclei having mass number between 100 and 200 we should use the heavy nucleus of mass number between 200 and 260. So that it will get split into two lighter nuclei having mass number between 100 and 200.
So, Fusion of two nuclei with mass number lying in the range of $50 < A < 100$ will release energy and fission of a nucleus lying in the mass range $200 < A < 260$ will release energy when broken into two equal fragments
Hence the correct answer is option (C) Both (A) and (B)
Note: It is a tricky question. Don’t get confused by the question. It is said that the nucleus having mass numbers between 100 and 200 hundred have large binding energy, which means the product of the fission and fusion process should have the large binding energy to release large amounts of energy.
Complete step by step answer:
When two lighter nuclei combine to form a stable nucleus, some mass of proton and neutron disappears, similarly when a nucleus get split into two lighter nuclei, here also some mass of proton and neutron get disappeared, the mass that disappears is converted into an equivalent amount of energy which is known as the binding energy of the nucleus
Nuclear fusion is the process of combining two or more nuclei to form a heavy nucleus. During this process a huge amount of energy is released.
Nuclear fission is the process of splitting an unstable nucleus to get two stable nuclei. During this process a huge amount of energy is released.
From the given graph we can see that the nucleus with mass number from \[100{\text{ }}to{\text{ }}200\] has a large binding energy.
So, if two lighter nuclei combine to form a stable nucleus of mass number between \[100{\text{ }}and{\text{ }}200\] then it will release large energy since it has large binding energy.
And also if the heavy nucleus splits into two lighter nuclei having mass number between \[100{\text{ }}and{\text{ }}200\] then it will release large energy since it has large binding energy.
Which means the nucleus formed by fusion should have mass number between \[100{\text{ }}and{\text{ }}200\] and the lighter nuclei formed by fission process should have mass number between \[100{\text{ }}and{\text{ }}200\] to release large amount of energy.
To form a nucleus having mass number between 100 and 200 we should use the lighter nuclei of mass number between 51 and 100. So that it will combine and the mass numbers of the two nuclei will get added and the resultant mass number will lie between 100 and 200.
To get two lighter nuclei having mass number between 100 and 200 we should use the heavy nucleus of mass number between 200 and 260. So that it will get split into two lighter nuclei having mass number between 100 and 200.
So, Fusion of two nuclei with mass number lying in the range of $50 < A < 100$ will release energy and fission of a nucleus lying in the mass range $200 < A < 260$ will release energy when broken into two equal fragments
Hence the correct answer is option (C) Both (A) and (B)
Note: It is a tricky question. Don’t get confused by the question. It is said that the nucleus having mass numbers between 100 and 200 hundred have large binding energy, which means the product of the fission and fusion process should have the large binding energy to release large amounts of energy.
Recently Updated Pages
JEE Main 2025-26 Mock Tests: Free Practice Papers & Solutions

JEE Main 2025-26 Experimental Skills Mock Test – Free Practice

JEE Main 2025-26 Electronic Devices Mock Test: Free Practice Online

JEE Main 2025-26 Atoms and Nuclei Mock Test – Free Practice Online

JEE Main 2025-26: Magnetic Effects of Current & Magnetism Mock Test

JEE Main Mock Test 2025: Properties of Solids and Liquids

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Physics Question Paper 2026: Download SET-wise PDF with Answer Key & Analysis

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Class 12 Physics Question Paper Set 3 (55/2/3) 2025: PDF, Answer Key & Solutions

CBSE Class 12 Physics Question Paper Set 3 (55/1/3) 2025 – PDF, Solutions & Analysis

CBSE Class 12 Physics Set 2 (55/2/2) 2025 Question Paper & Solutions




