
Assertion: The para-isomers have higher melting point as compared to their ortho and meta-isomers.
Reason: It is due to symmetry of para-isomers that fits in crystal lattice better as compared to ortho and meta-isomers.
(A) Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
(B) Both Assertion and Reason are correct and Reason is not the correct explanation for Assertion
(C) Assertion is correct but Reason is incorrect
(D) Both Assertion and Reason are incorrect
Answer
232.8k+ views
Hint: There is a general rule that in crystalline form, symmetrical molecules exhibit higher melting temperatures as compared to molecules with similar structure having lower symmetry.
Complete step by step answer: Let us consider the simple aromatic hydrocarbon and number the carbon positions as shown below:
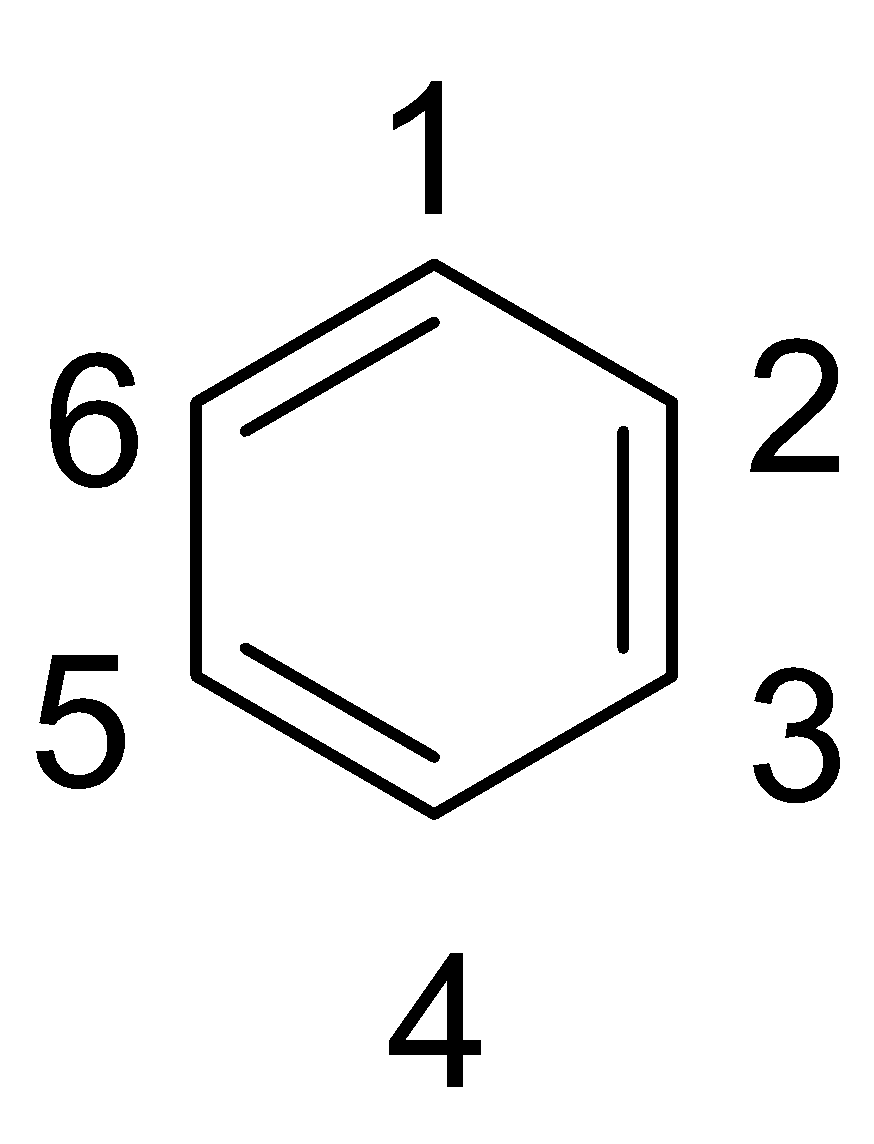
If two substituents occupy the positions 1 and 2, then it is called ortho substitution and the isomer is called the ortho-isomer. If two substituents occupy the positions 1 and 3, then it is called meta substitution and the isomer is called the meta-isomer and if two substituents occupy the positions 1 and 4, then it is called para substitution and the isomer is called the para-isomer.
Let us consider the substituent to be X. Then the isomers will be:
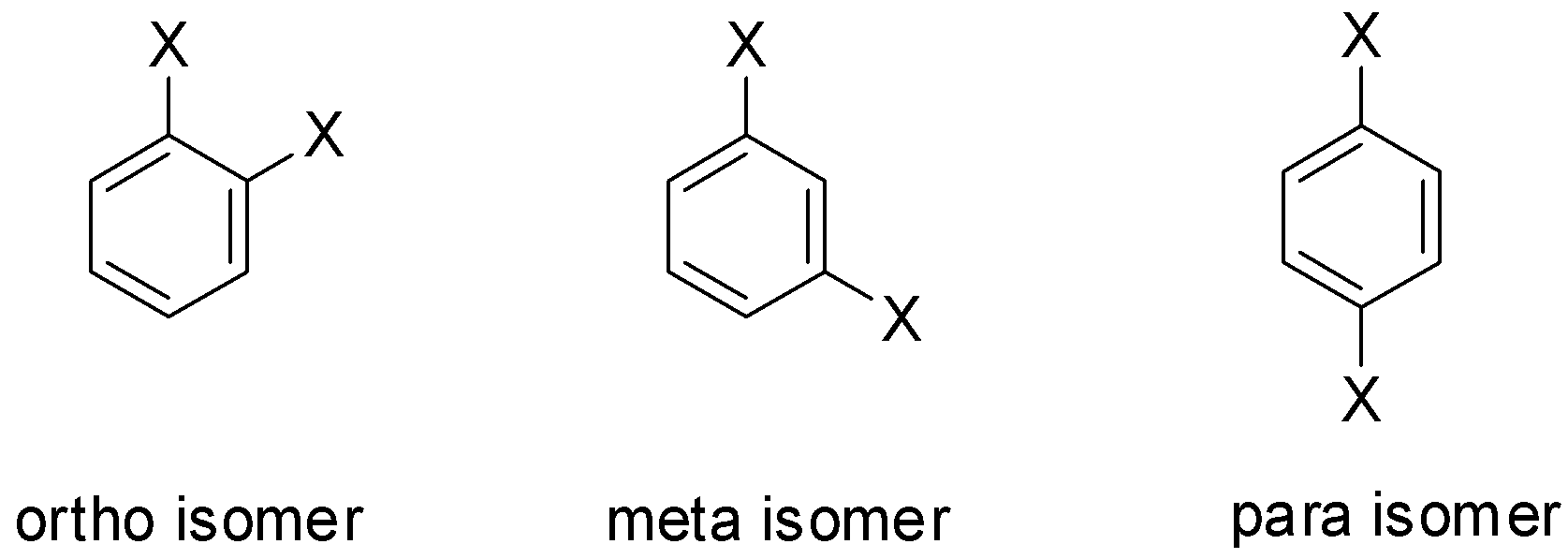
The para-isomer has symmetrical structure and so it can easily fit into a crystal lattice. As a result, the intermolecular forces of attraction are very strong in the para-isomer and so the amount of energy which is required to break the crystal lattice of the para-isomer is also very large. Or in other words, more energy is required to melt the para-isomer and hence it has a very high melting point.
On the other hand, the ortho-isomer and the meta-isomer are not so symmetrical in comparison to the para-isomer and hence they do not form a very closely packed crystal lattice. As a result, the intermolecular forces of attraction are not very strong as compared to those in the para-isomer. Thus, the ortho-isomer and the meta-isomer have very weak forces of attraction and so the amount of energy required to break down the crystal lattices of the ortho-isomer and the meta-isomer is also very less in comparison to that in the para-isomer. This means that the energy required to melt the crystal lattices of the ortho and meta-isomers is smaller than that needed for the para-isomer. So they will have lower melting points than the para-isomer.
Hence, the melting point of the para-isomer is high as compared to the ortho and meta-isomers because the para-isomer is more symmetrical than the ortho and meta-isomers. So, both assertion and reason for the given question are correct and the reason is the correct explanation for assertion. So the correct option is option A.
Note: Molecular packing affects the melting point order. A substance will have high melting point if its molecules are more tightly packed and lower melting point if they are not packed well.
Complete step by step answer: Let us consider the simple aromatic hydrocarbon and number the carbon positions as shown below:

If two substituents occupy the positions 1 and 2, then it is called ortho substitution and the isomer is called the ortho-isomer. If two substituents occupy the positions 1 and 3, then it is called meta substitution and the isomer is called the meta-isomer and if two substituents occupy the positions 1 and 4, then it is called para substitution and the isomer is called the para-isomer.
Let us consider the substituent to be X. Then the isomers will be:

The para-isomer has symmetrical structure and so it can easily fit into a crystal lattice. As a result, the intermolecular forces of attraction are very strong in the para-isomer and so the amount of energy which is required to break the crystal lattice of the para-isomer is also very large. Or in other words, more energy is required to melt the para-isomer and hence it has a very high melting point.
On the other hand, the ortho-isomer and the meta-isomer are not so symmetrical in comparison to the para-isomer and hence they do not form a very closely packed crystal lattice. As a result, the intermolecular forces of attraction are not very strong as compared to those in the para-isomer. Thus, the ortho-isomer and the meta-isomer have very weak forces of attraction and so the amount of energy required to break down the crystal lattices of the ortho-isomer and the meta-isomer is also very less in comparison to that in the para-isomer. This means that the energy required to melt the crystal lattices of the ortho and meta-isomers is smaller than that needed for the para-isomer. So they will have lower melting points than the para-isomer.
Hence, the melting point of the para-isomer is high as compared to the ortho and meta-isomers because the para-isomer is more symmetrical than the ortho and meta-isomers. So, both assertion and reason for the given question are correct and the reason is the correct explanation for assertion. So the correct option is option A.
Note: Molecular packing affects the melting point order. A substance will have high melting point if its molecules are more tightly packed and lower melting point if they are not packed well.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




