
Aspirin is known as:
(A) Acetylsalicylic acid
(B) Phenyl salicylate
(C) Acetyl salicylate
(D) Methylsalicylic acid
Answer
233.1k+ views
Hint: The structure of aspirin consists of benzene ring ${ C }_{ 6 }{ H }_{ 6 }$ with carboxylic acid (COOH) and an ester as a functional group. It is prepared by Kolbe Schmitt reaction by treating phenol with an alkali. After that, an anhydride and sulphuric acid is used for the formation of aspirin.
Complete step-by-step answer:
The chemical name of aspirin is ${ 2 }$-acetoxy benzoic acid. Aspirin, otherwise called acetylsalicylic corrosive (ASA), is a drug used to treat torment, fever, and irritation. Explicit provocative conditions in which it is utilized incorporate Kawasaki malady, pericarditis, and rheumatic fever.
Anti-inflammatory medicine given soon after a coronary episode diminishes the danger of death. Ibuprofen is additionally utilized long haul to help forestall coronary episodes, strokes, and blood clumps, in individuals at high hazard. Headache medicine may likewise diminish the danger of specific sorts of disease, especially colorectal malignant growth. For agony or fever, impacts regularly start inside ${ 30 }$ minutes.
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID) and works similarly to other NSAIDs but it is also antiplatelet and suppresses the normal functioning of platelets.
Acetylsalicylic acid (aspirin) is prepared from phenol by the first carboxylation of sodium phenoxide by Kolbe-Schmitt reaction to form salicylic acid followed by acetylation.
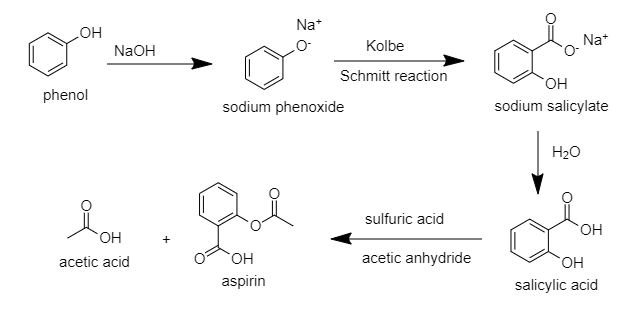
Hence, the correct option is A.
Additional Information:
When our body is in stress it triggers a reaction to the cellular level. This reaction often ends with the generation of a class of compounds called prostaglandins. It causes pain and fever in the body. Aspirin prevents the generation of prostaglandins by inhibiting the enzyme which produces them (cyclooxygenase), thus relieving pain, fever, and inflammation.
Note: The possibility to make a mistake is that you may choose option C. But aspirin is acetylsalicylic acid, not acetylsalicylate, and it is an analgesic.
Complete step-by-step answer:
The chemical name of aspirin is ${ 2 }$-acetoxy benzoic acid. Aspirin, otherwise called acetylsalicylic corrosive (ASA), is a drug used to treat torment, fever, and irritation. Explicit provocative conditions in which it is utilized incorporate Kawasaki malady, pericarditis, and rheumatic fever.
Anti-inflammatory medicine given soon after a coronary episode diminishes the danger of death. Ibuprofen is additionally utilized long haul to help forestall coronary episodes, strokes, and blood clumps, in individuals at high hazard. Headache medicine may likewise diminish the danger of specific sorts of disease, especially colorectal malignant growth. For agony or fever, impacts regularly start inside ${ 30 }$ minutes.
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID) and works similarly to other NSAIDs but it is also antiplatelet and suppresses the normal functioning of platelets.
Acetylsalicylic acid (aspirin) is prepared from phenol by the first carboxylation of sodium phenoxide by Kolbe-Schmitt reaction to form salicylic acid followed by acetylation.

Hence, the correct option is A.
Additional Information:
When our body is in stress it triggers a reaction to the cellular level. This reaction often ends with the generation of a class of compounds called prostaglandins. It causes pain and fever in the body. Aspirin prevents the generation of prostaglandins by inhibiting the enzyme which produces them (cyclooxygenase), thus relieving pain, fever, and inflammation.
Note: The possibility to make a mistake is that you may choose option C. But aspirin is acetylsalicylic acid, not acetylsalicylate, and it is an analgesic.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




