
Analyze the energy diagram below for a reaction.
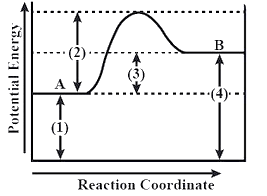
Which area on the graph is not directly related to the overall reaction being exothermic or endothermic?
(A) 1
(B) 2
(C) 3
(D) 4
Answer
232.8k+ views
Hint: For this problem, firstly we have to study the activation energy and about exothermic and endothermic reactions. So that we can determine the correct area which does not directly depend on the overall reaction.
Complete step by step solution:
-In the given question, we have to explain which area on the graph does not show a direct relationship with the overall reaction.
-The given graph represents the type reaction whether it is in forwarding direction or a backward direction or whether it is an exothermic or endothermic reaction.
-Now, the activation energy is defined as the energy which is required for an atom to initiate the process of a chemical reaction.
-This activation energy helps the reaction to initiate in the forward direction that is from reactant to the product.
-In between the reaction completion, there is a state known as transition state which has higher energy than the reactant and product.
-In the given graph, part A represents the energy of reactant i.e. ${{\text{E}}_{\text{A}}}$which will proceed into the chemical reaction to form the product B i.e. ${{\text{E}}_{\text{B}}}$.
-Whereas the position (3) shows the difference between the energy of reactant and product which is given by: ${{\text{E}}_{\text{B}}}\text{ - }{{\text{E}}_{\text{A}}}$.
-So, we can say these three positions are directly related to the overall reaction because if the energy of A will be more than the energy of B then the reaction will be exothermic and vice versa.
-But the position (2) represents the activation energy which does not have a direct relationship with overall reaction because it only helps in initiating the process and doesn't tell whether it is exothermic or endothermic.
Therefore, option (B) is the correct answer.
Note: The transition state formed is unstable because the molecules start to break down and convert into the product. Moreover, the exothermic reaction yields heat and increases the temperature.
Complete step by step solution:
-In the given question, we have to explain which area on the graph does not show a direct relationship with the overall reaction.
-The given graph represents the type reaction whether it is in forwarding direction or a backward direction or whether it is an exothermic or endothermic reaction.
-Now, the activation energy is defined as the energy which is required for an atom to initiate the process of a chemical reaction.
-This activation energy helps the reaction to initiate in the forward direction that is from reactant to the product.
-In between the reaction completion, there is a state known as transition state which has higher energy than the reactant and product.
-In the given graph, part A represents the energy of reactant i.e. ${{\text{E}}_{\text{A}}}$which will proceed into the chemical reaction to form the product B i.e. ${{\text{E}}_{\text{B}}}$.
-Whereas the position (3) shows the difference between the energy of reactant and product which is given by: ${{\text{E}}_{\text{B}}}\text{ - }{{\text{E}}_{\text{A}}}$.
-So, we can say these three positions are directly related to the overall reaction because if the energy of A will be more than the energy of B then the reaction will be exothermic and vice versa.
-But the position (2) represents the activation energy which does not have a direct relationship with overall reaction because it only helps in initiating the process and doesn't tell whether it is exothermic or endothermic.
Therefore, option (B) is the correct answer.
Note: The transition state formed is unstable because the molecules start to break down and convert into the product. Moreover, the exothermic reaction yields heat and increases the temperature.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




