
An oxidizing agent is a substance which can
A.Accept electrons
B.Donate electrons
C.Accept protons
D.Donate protons
Answer
232.5k+ views
Hint: Basically, an oxidizing agent is a chemical species that tends to oxidize other substances. It causes an increase in the oxidation state of the substance by making it lose electrons. Generally, it takes the electrons towards itself and thus it gains electrons and gets reduced.
Complete step by step answer:
An oxidizing agent, also known as an oxidizer or oxidant is a chemical compound that readily transfers oxygen atoms in a redox reaction. Basically, it helps in the oxidation while getting reduced by gaining hydrogen and giving oxygen or gaining electrons form the other reactants. Some of the common examples of oxidizing agents are halogens such as chlorine and fluorine, oxygen and hydrogen peroxide.
Now, oxidizing agents can be defined in two different ways:
1.As an electron acceptor- These are the chemical substances whose atoms remove at least one electron from another atom in a chemical reaction. Basically, oxidizing agents are the reactants that undergo reduction in redox reactions. An illustration is as shown:
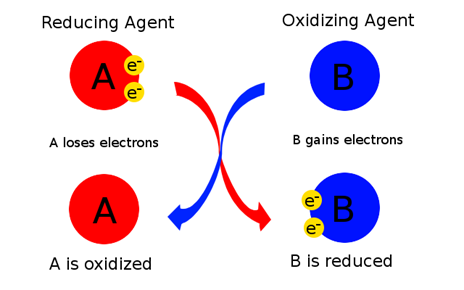
We can see that substance A undergoes oxidation, resulting in an increase in oxidation number whereas substance B becomes smaller as it gains electrons by undergoing reduction.
2.As an atom transferring substance- It is a substance that transfers at least one electronegative atom to a chemical species in a chemical reaction and the transferred atom is typically an oxygen atom. The example is as shown:
\[F{e_2}{O_3} + 3CO \to 2Fe + 3C{O_2}\]
In this case, the $F{e_2}{O_3}$ molecule acts as an oxidizer by transferring the electronegative oxygen atom to carbon monoxide molecule.
Therefore, an oxidizing agent is a substance which can accept electrons.
Hence, option A is correct.
Note:Oxidizing agents have various commercial and industrial applications. They are used in bleaching of fabrics, purification of water, storage of energy in batteries, vulcanization of rubber. Moreover, they are also vital to many biological processes such as metabolism and photosynthesis.
Complete step by step answer:
An oxidizing agent, also known as an oxidizer or oxidant is a chemical compound that readily transfers oxygen atoms in a redox reaction. Basically, it helps in the oxidation while getting reduced by gaining hydrogen and giving oxygen or gaining electrons form the other reactants. Some of the common examples of oxidizing agents are halogens such as chlorine and fluorine, oxygen and hydrogen peroxide.
Now, oxidizing agents can be defined in two different ways:
1.As an electron acceptor- These are the chemical substances whose atoms remove at least one electron from another atom in a chemical reaction. Basically, oxidizing agents are the reactants that undergo reduction in redox reactions. An illustration is as shown:

We can see that substance A undergoes oxidation, resulting in an increase in oxidation number whereas substance B becomes smaller as it gains electrons by undergoing reduction.
2.As an atom transferring substance- It is a substance that transfers at least one electronegative atom to a chemical species in a chemical reaction and the transferred atom is typically an oxygen atom. The example is as shown:
\[F{e_2}{O_3} + 3CO \to 2Fe + 3C{O_2}\]
In this case, the $F{e_2}{O_3}$ molecule acts as an oxidizer by transferring the electronegative oxygen atom to carbon monoxide molecule.
Therefore, an oxidizing agent is a substance which can accept electrons.
Hence, option A is correct.
Note:Oxidizing agents have various commercial and industrial applications. They are used in bleaching of fabrics, purification of water, storage of energy in batteries, vulcanization of rubber. Moreover, they are also vital to many biological processes such as metabolism and photosynthesis.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




