
An ideal gas is trapped between the mercury column and the closed end of a narrow vertical tube containing the column. The upper end of the tube is open to the atmosphere. The atmospheric pressure is equal to 76cm of Hg. The lengths of mercury column and trapped air column are 20 cm and 43 cm respectively. What will be the length of the air column if the tube is tiled in the vertical plane with an of ${60^ \circ }$? Assume the temperature to be constant.
Answer
232.8k+ views
Hint: In this question we have to do the analysis of pressure at the bottom of the flask. Doing so in the two given conditions will give us the new length of air columns.
Complete step by step solution:
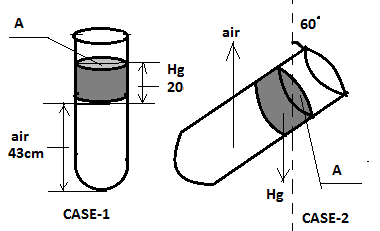
Case 1: Before tilting;
Let the pressure of trapped air be ${P_1}$
This will be the pressure at the air-mercury interface.
Also atmospheric pressure ( ${P_0}$ ) = 76 cm of Hg.
Now at point on the air-mercury interface the pressure will be;
${P_0} + {P_{Hg}} = {P_1}$
${P_1} = 76 + 20 = 96$ (Equation: 1)
Now since air is an ideal gas it will have uniform pressure throughout it. So the pressure at the bottom of the flask will be ${P_1} = 96$
Let the area of the tube be A
So applying gas law we get;
${P_1}V = nRT$
Now we all know, $V = Al$
And $l = 43cm$
Thus $ \Rightarrow 96(A \times 43) = nRT$ (Equation: 2)
Now let us consider case 2 (after tilling);
The pressure at the air mercury interface ( ${P_2}$ ) is given by;
${P_2} = {P_0} + {P_{Hg}}\cos ({60^ \circ })$
Thus, $ \Rightarrow {P_2} = 79 + \dfrac{{20}}{2} = 86$
Now applying gas law we get;
${P_2}V = nRT$
$ \Rightarrow 86(Al') = nRT$ (Equation: 3) (here, $l'$ is the new length of air column)
From equation 2 and equation 3 we get;
$ \Rightarrow 96 \times 43A = 86Al'$
$\therefore l' = \dfrac{{96 \times 43}}{{86}} = 48cm$
Therefore, the new length is 48 cm.
Note:The volume is changing and hence the area is constant.
If the lid will be closed the volume won’t change and hence the area will change.
The pressure balancing should be done carefully.
Complete step by step solution:

Case 1: Before tilting;
Let the pressure of trapped air be ${P_1}$
This will be the pressure at the air-mercury interface.
Also atmospheric pressure ( ${P_0}$ ) = 76 cm of Hg.
Now at point on the air-mercury interface the pressure will be;
${P_0} + {P_{Hg}} = {P_1}$
${P_1} = 76 + 20 = 96$ (Equation: 1)
Now since air is an ideal gas it will have uniform pressure throughout it. So the pressure at the bottom of the flask will be ${P_1} = 96$
Let the area of the tube be A
So applying gas law we get;
${P_1}V = nRT$
Now we all know, $V = Al$
And $l = 43cm$
Thus $ \Rightarrow 96(A \times 43) = nRT$ (Equation: 2)
Now let us consider case 2 (after tilling);
The pressure at the air mercury interface ( ${P_2}$ ) is given by;
${P_2} = {P_0} + {P_{Hg}}\cos ({60^ \circ })$
Thus, $ \Rightarrow {P_2} = 79 + \dfrac{{20}}{2} = 86$
Now applying gas law we get;
${P_2}V = nRT$
$ \Rightarrow 86(Al') = nRT$ (Equation: 3) (here, $l'$ is the new length of air column)
From equation 2 and equation 3 we get;
$ \Rightarrow 96 \times 43A = 86Al'$
$\therefore l' = \dfrac{{96 \times 43}}{{86}} = 48cm$
Therefore, the new length is 48 cm.
Note:The volume is changing and hence the area is constant.
If the lid will be closed the volume won’t change and hence the area will change.
The pressure balancing should be done carefully.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




