
Amongst \[Cl{{F}_{3}}\], \[B{{F}_{3}}\], \[~N{{H}_{3}}\] molecule the one with non-planar geometry is:
A. \[Cl{{F}_{3}}\]
B. \[~N{{H}_{3}}\]
C. \[B{{F}_{3}}\]
D. None of these
Answer
232.8k+ views
Hint: Geometry of any molecule is defined as the structure formation considering or including the repulsive effect of lone pairs on other atoms present in a molecule (if present) as per VSEPR theory. Whereas the shape of any molecule gives us structure without considering or can say neglecting the effect of lone pairs on other atoms of a molecule.
Complete Step by Step Solution:
Atomic number of chlorine is 17, thus has 7 electron remains in outermost shell (M shell) having electronic configuration \[3s{}^\text{2},\text{ }3{{p}^{5}}\]. From five electrons (excluding lone pair electrons) filled in p orbital, one electron will jump in d orbital (empty orbital), so that it can form three bonds with three fluorine.
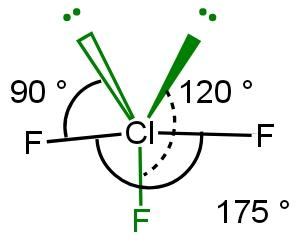
There are two lone pairs (one from 3s orbital and second lone pair from 3p orbital and). As lone pair-lone pair repulsion is more than other repulsion thus, both lone pair will take equatorial position so that form 90 degree angle with only two fluorine (and not with three fluorine, in case if lone pair occupy axial position) and 120 degree angle with third fluorine. From this lone pair maintains good distance with three fluorine atoms to reduce repulsion. Two fluorine atoms will occupy axial position and one fluoride will occupy equatorial position to make repulsion minimum. The angle between two equatorial fluorine atoms is 175 degrees. This will form trigonal bipyramidal structure such as
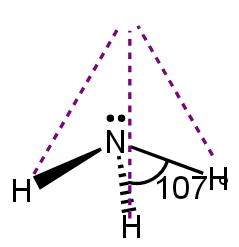
In \[~N{{H}_{3}}\](\[s{{p}^{3}}\]), there is one lone pair on nitrogen present (occupy equatorial position) repelling the entire three bond (between hydrogen and nitrogen) at around 107 degree. This gives a trigonal pyramidal structure of ammonia.
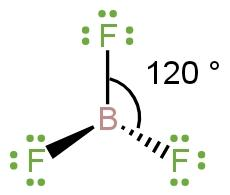
Whereas in \[B{{F}_{3}}\] (sp² hybridization) there is no lone pair thus, there will only be bond pair-bond pair repulsion. Two bonds are already in plane and the third bond will also be in plane to minimise the effect of repulsion forming tribunal planar structure with 120 ° bond angle between fluorine atoms.
Thus, the correct option is C.
Note: Equatorial plane is a horizontal plane while an axial plane is vertical plane and thus perpendicular to the equatorial plane. Lone pair prefers to be at equatorial position so that surrounded by less number of other atoms and reduce repulsion makes compound more stable.
Complete Step by Step Solution:
Atomic number of chlorine is 17, thus has 7 electron remains in outermost shell (M shell) having electronic configuration \[3s{}^\text{2},\text{ }3{{p}^{5}}\]. From five electrons (excluding lone pair electrons) filled in p orbital, one electron will jump in d orbital (empty orbital), so that it can form three bonds with three fluorine.
There are two lone pairs (one from 3s orbital and second lone pair from 3p orbital and). As lone pair-lone pair repulsion is more than other repulsion thus, both lone pair will take equatorial position so that form 90 degree angle with only two fluorine (and not with three fluorine, in case if lone pair occupy axial position) and 120 degree angle with third fluorine. From this lone pair maintains good distance with three fluorine atoms to reduce repulsion. Two fluorine atoms will occupy axial position and one fluoride will occupy equatorial position to make repulsion minimum. The angle between two equatorial fluorine atoms is 175 degrees. This will form trigonal bipyramidal structure such as

In \[~N{{H}_{3}}\](\[s{{p}^{3}}\]), there is one lone pair on nitrogen present (occupy equatorial position) repelling the entire three bond (between hydrogen and nitrogen) at around 107 degree. This gives a trigonal pyramidal structure of ammonia.

Whereas in \[B{{F}_{3}}\] (sp² hybridization) there is no lone pair thus, there will only be bond pair-bond pair repulsion. Two bonds are already in plane and the third bond will also be in plane to minimise the effect of repulsion forming tribunal planar structure with 120 ° bond angle between fluorine atoms.

Thus, the correct option is C.
Note: Equatorial plane is a horizontal plane while an axial plane is vertical plane and thus perpendicular to the equatorial plane. Lone pair prefers to be at equatorial position so that surrounded by less number of other atoms and reduce repulsion makes compound more stable.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




