
Among the following, which should have the highest r.m.s speed at the same temperature?
A.${\text{S}}{{\text{O}}_{\text{2}}}$
B.${\text{C}}{{\text{O}}_{\text{2}}}$
C.${{\text{O}}_{\text{2}}}$
D.${{\text{H}}_{\text{2}}}$
Answer
242.7k+ views
Hint: To answer this question, you should recall the concept of root-mean-square speed of gas. Study the dependence of factors which affect this root mean square speed. Now use the factors to find the answer to this question.
The formula used:
${{\text{v}}_{{\text{rms}}}} = \sqrt {\dfrac{{{\text{3RT}}}}{{\text{M}}}} $ where ${{\text{v}}_{{\text{rms}}}}$=Root mean square speed, ${\text{R}}$= Universal gas constant, ${\text{T}}$= Temperature and ${\text{M}}$ is the Molar Mass of gas ---(i)
Complete Step by step solution:
We know that root mean square speed t is the square root of the average velocity-squared of the molecules in a gas. You can see that it takes into account both molecular weight and temperature, these factors directly affect the kinetic energy of a gas.
From equation (i) we can conclude that
${{\text{v}}_{{\text{rms}}}} \propto \dfrac{1}{{\text{M}}}$.
The molar mass will directly affect the root mean square speed.
The molar mass of each gas will be ${\text{S}}{{\text{O}}_{\text{2}}}$ = 64 g , ${\text{C}}{{\text{O}}_{\text{2}}}$ = 44 g, ${{\text{O}}_{\text{2}}}$ = 32 g, ${{\text{H}}_{\text{2}}}$ = 2 g
Out of the given options ${{\text{H}}_{\text{2}}}$ has the smallest molar mass thus, the highest r.m.s speed.
Therefore, we can conclude that the correct answer to this question is D.
Additional information: At 'higher temperature' and 'lower pressure', a gas behaves like an ideal gas, as the potential energy due to intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the space between them.
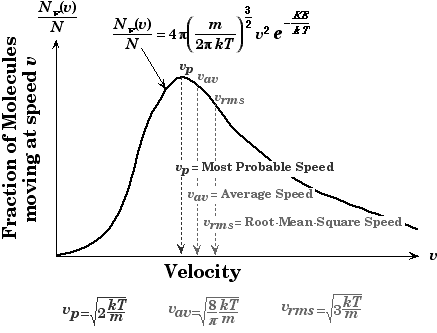
Note: Along with different speeds you should know the concept of Maxwell-Boltzmann equation. The Maxwell-Boltzmann equation helps define the distribution of speeds for gas at various temperatures. From this distribution graph function, the most probable speed, the average speed, and the root-mean-square speed can be derived. The most probable speed is the speed most likely to be possessed by any molecule in the system.
The formula used:
${{\text{v}}_{{\text{rms}}}} = \sqrt {\dfrac{{{\text{3RT}}}}{{\text{M}}}} $ where ${{\text{v}}_{{\text{rms}}}}$=Root mean square speed, ${\text{R}}$= Universal gas constant, ${\text{T}}$= Temperature and ${\text{M}}$ is the Molar Mass of gas ---(i)
Complete Step by step solution:
We know that root mean square speed t is the square root of the average velocity-squared of the molecules in a gas. You can see that it takes into account both molecular weight and temperature, these factors directly affect the kinetic energy of a gas.
From equation (i) we can conclude that
${{\text{v}}_{{\text{rms}}}} \propto \dfrac{1}{{\text{M}}}$.
The molar mass will directly affect the root mean square speed.
The molar mass of each gas will be ${\text{S}}{{\text{O}}_{\text{2}}}$ = 64 g , ${\text{C}}{{\text{O}}_{\text{2}}}$ = 44 g, ${{\text{O}}_{\text{2}}}$ = 32 g, ${{\text{H}}_{\text{2}}}$ = 2 g
Out of the given options ${{\text{H}}_{\text{2}}}$ has the smallest molar mass thus, the highest r.m.s speed.
Therefore, we can conclude that the correct answer to this question is D.
Additional information: At 'higher temperature' and 'lower pressure', a gas behaves like an ideal gas, as the potential energy due to intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the space between them.
Note: Along with different speeds you should know the concept of Maxwell-Boltzmann equation. The Maxwell-Boltzmann equation helps define the distribution of speeds for gas at various temperatures. From this distribution graph function, the most probable speed, the average speed, and the root-mean-square speed can be derived. The most probable speed is the speed most likely to be possessed by any molecule in the system.

Recently Updated Pages
WBJEE 2026 Registration Started: Important Dates Eligibility Syllabus Exam Pattern

Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

CBSE Notes Class 11 Chemistry Chapter 8 - Organic Chemistry Some Basic Principles And Techniques - 2025-26




