
Among the following compounds\[{C_3}{H_7}N{H_2}\], \[N{H_3}\], \[C{H_3}N{H_2}\], \[{C_6}{H_5}N{H_2}\]and \[{C_2}{H_5}N{H_2}\], the least basic compound is?
A.\[{C_3}{H_7}N{H_2}\]\[\]
B.\[N{H_3}\]
C.\[C{H_3}N{H_2}\]
D.\[{C_6}{H_5}N{H_2}\]
E.\[{C_2}{H_5}N{H_2}\]
Answer
233.1k+ views
Hint: Basicity is the electron donor ability of compounds. Compounds having non-bonding electrons show basic character as they can donate their electrons. More donating power of electrons makes the compound more basic.
Complete Step by Step Solution:
All the compounds given in the question are basic because of the presence of nitrogen atoms in them which have one lone pair of electrons. But they have a difference in their basicity. The ones whose electrons are readily available for donation are more basic than the ones whose electrons are delocalised in the compound. Because if the electrons are delocalized, they are not readily available to donate.
From the given compounds, only \[{C_6}{H_5}N{H_2}\]i.e., aniline is the one where electrons are delocalised on the compound and its electron donation power decreases. Due to this, they are not readily available for donation and hence it is the least basic compound. After option D, Option E is less basic because of the pie bond of ethene on the adjacent position of amine. Here also, lone pairs of electrons of Nitrogen are delocalised in the compound. But, comparatively, it is less than aniline because of comparatively less delocalisation as it has less no. of pie bonds.
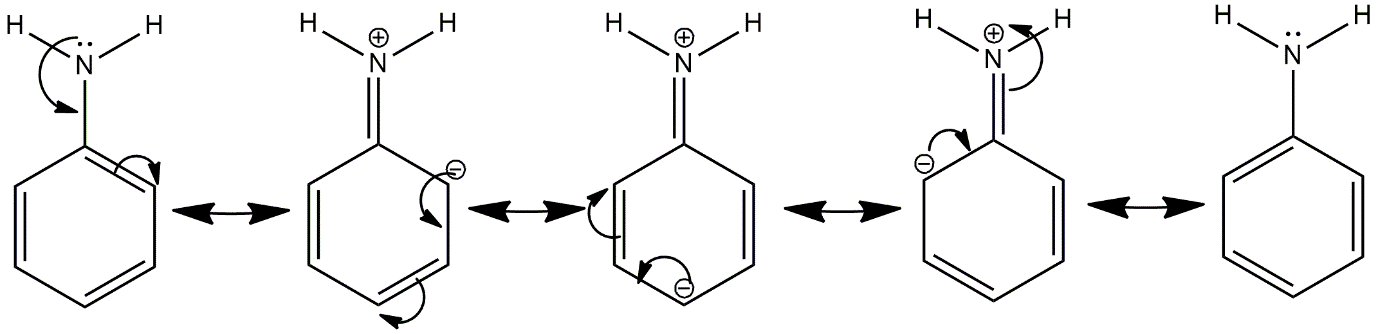
Image:Delocalisation of lone pair of electrons of Nitrogen in aniline compound.
So, option D is correct.
Note: Alkyl groups in electron-donating groups, increased the tendency of atoms to donate their electrons. So, they help in increasing the basicity of the compound. Basicity can be used to find compounds that can be easily protonated. So, the most basic compound is the one which gets protonated fast. Basic compounds donate their electrons and form bonds between them, in return, they become positively charged.
Complete Step by Step Solution:
All the compounds given in the question are basic because of the presence of nitrogen atoms in them which have one lone pair of electrons. But they have a difference in their basicity. The ones whose electrons are readily available for donation are more basic than the ones whose electrons are delocalised in the compound. Because if the electrons are delocalized, they are not readily available to donate.
From the given compounds, only \[{C_6}{H_5}N{H_2}\]i.e., aniline is the one where electrons are delocalised on the compound and its electron donation power decreases. Due to this, they are not readily available for donation and hence it is the least basic compound. After option D, Option E is less basic because of the pie bond of ethene on the adjacent position of amine. Here also, lone pairs of electrons of Nitrogen are delocalised in the compound. But, comparatively, it is less than aniline because of comparatively less delocalisation as it has less no. of pie bonds.

Image:Delocalisation of lone pair of electrons of Nitrogen in aniline compound.
So, option D is correct.
Note: Alkyl groups in electron-donating groups, increased the tendency of atoms to donate their electrons. So, they help in increasing the basicity of the compound. Basicity can be used to find compounds that can be easily protonated. So, the most basic compound is the one which gets protonated fast. Basic compounds donate their electrons and form bonds between them, in return, they become positively charged.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




