
Aldehydes are isomeric with
(A) Ketones
(B) Ethers
(C) Alcohols
(D) Fatty acids
Answer
232.8k+ views
Hint: All the given compounds in the question are organic compounds which differ in functional group. So, for these types of questions, the isomerism should be functional constitutional isomers i.e., the molecular formula is the same but the compounds differ in the functional group attached within the compound.
Complete Step by Step Solution:
Aldehydes: These are the organic compounds which consist of a carbon chain that may be aliphatic, aromatic or hydrogen atom bonded to a terminal carbon consisting of double bond oxygen and single bond hydrogen.
The structure of an aldehyde can be diagrammatically represented as follows:
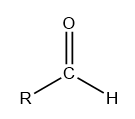
Where R can be a hydrogen atom, aliphatic or aromatic ring.
Ketones: These are the organic compounds in which the carbon atom is bonded to oxygen via double bond and both the terminal bonds of the carbon atom are attached to a carbon chain that may be aromatic or aliphatic.
The structure of a ketone can be diagrammatically represented as follows:
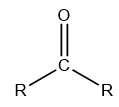
Ethers: These compounds belong to the class of organic compounds in which two carbon chains are connected to each other via oxygen atoms. An ether is generally represented as ${\rm{R}} - {\rm{O}} - {\rm{R}}$
Alcohols: These compounds belong to the class of organic compounds in which a carbon chain (aliphatic or aromatic) is bonded to the hydroxide group. The general structure of an alcohol is ${\rm{R}} - {\rm{OH}}$.
Fatty acids: These compounds are the type of organic compounds which consist of a long aliphatic chain of carbon bonded to a carboxylic group. The general structure of a fatty acid is $C{H_3}{(C{H_2})_n}COOH$, where n is usually an even number and lies between $2 - 28$.
Among the given options, it is clear that alcohols and ether have one carbon less as compared to aldehyde and thus cannot be isomeric while in fatty acid, there is an extra oxygen atom present in the structure as compared to aldehyde, so this option is eliminated as well.
Thus, the aldehydes are isomeric in nature with ketones showing functional isomerism. An example showing the functional isomerism in aldehydes and ketones is as follows:
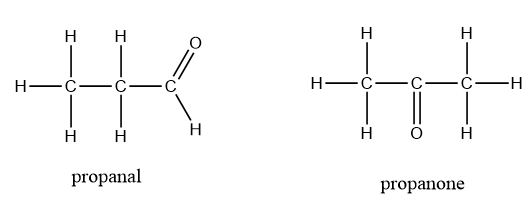
Therefore, option (A) is the correct answer.
Note: It is important to note that in organic chemistry, the functional groups which are isomeric to each other i.e., they have similar molecular structure but the functional groups attached to the compound are not same and differ in physical and chemical properties. The following are the functional groups which are generally isomeric to each other:
1. Alcohols and ethers
2. Aldehydes and ketones
3. Carboxylic acids and esters.
Complete Step by Step Solution:
Aldehydes: These are the organic compounds which consist of a carbon chain that may be aliphatic, aromatic or hydrogen atom bonded to a terminal carbon consisting of double bond oxygen and single bond hydrogen.
The structure of an aldehyde can be diagrammatically represented as follows:

Where R can be a hydrogen atom, aliphatic or aromatic ring.
Ketones: These are the organic compounds in which the carbon atom is bonded to oxygen via double bond and both the terminal bonds of the carbon atom are attached to a carbon chain that may be aromatic or aliphatic.
The structure of a ketone can be diagrammatically represented as follows:

Ethers: These compounds belong to the class of organic compounds in which two carbon chains are connected to each other via oxygen atoms. An ether is generally represented as ${\rm{R}} - {\rm{O}} - {\rm{R}}$
Alcohols: These compounds belong to the class of organic compounds in which a carbon chain (aliphatic or aromatic) is bonded to the hydroxide group. The general structure of an alcohol is ${\rm{R}} - {\rm{OH}}$.
Fatty acids: These compounds are the type of organic compounds which consist of a long aliphatic chain of carbon bonded to a carboxylic group. The general structure of a fatty acid is $C{H_3}{(C{H_2})_n}COOH$, where n is usually an even number and lies between $2 - 28$.
Among the given options, it is clear that alcohols and ether have one carbon less as compared to aldehyde and thus cannot be isomeric while in fatty acid, there is an extra oxygen atom present in the structure as compared to aldehyde, so this option is eliminated as well.
Thus, the aldehydes are isomeric in nature with ketones showing functional isomerism. An example showing the functional isomerism in aldehydes and ketones is as follows:

Therefore, option (A) is the correct answer.
Note: It is important to note that in organic chemistry, the functional groups which are isomeric to each other i.e., they have similar molecular structure but the functional groups attached to the compound are not same and differ in physical and chemical properties. The following are the functional groups which are generally isomeric to each other:
1. Alcohols and ethers
2. Aldehydes and ketones
3. Carboxylic acids and esters.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding How a Current Loop Acts as a Magnetic Dipole

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Atomic Structure for Beginners

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)




