
According to Markonikov’s rule during formation of alkyl halide form alkene and halo acid which product is more stable?
A. The product which contains primary carbocation.
B. The product which contains secondary carbocation.
C. The product which contains tertiary carbocation.
D. None of these.
Answer
233.1k+ views
Hint: Markonikov’s rule is applicable only for unsymmetrical alkene. Alkenes undergo electrophilic addition reactions. Additional reactions are very common in case of alkenes because of their unsaturation property. The additional reactions chemically take place by formation of carbocation.
Complete step by step solution:
According to Markonikov’s rule: During unsymmetrical alkene, the negative part of the attacking reagent goes to the carbon carrying less number of hydrogen atoms and positive part goes to the other carbon atom. In other words the addition of hydrogen takes place in the highly unsaturated carbon atom of alkene.
For example: Addition of$HBr$to unsymmetrical alkene. When $HBr$is added to an alkene which is unsymmetrical, two types of products are formed as given below:
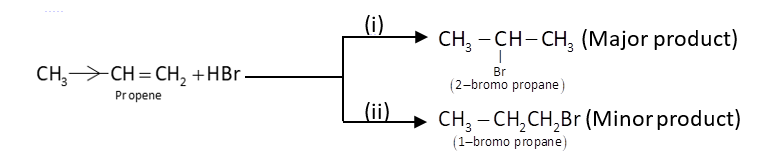
Mechanism: It is an electrophilic addition which is favoured by the formation of more stable carbonium ion intermediate.
(i) $HBr$undergoes ionisation in the presence of propene molecule to give electrophile $\left( {{H^ + }} \right)$and nucleophile $\left( {B{r^ - }} \right)$.
$H - Br\xrightarrow{{}}\mathop {{H^ \oplus }}\limits_{\left( {electrophile} \right)} + \mathop {B{r^ - }}\limits_{\left( {nucleophile} \right)} $
(ii) ${H^ \oplus }$attacks the double bond to produce a more stable secondary carbocation ion.
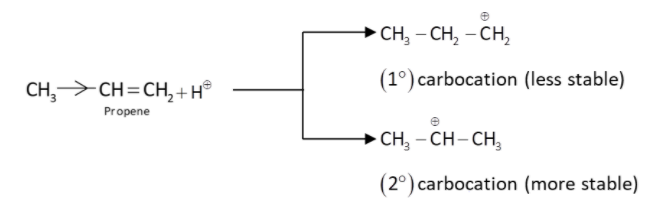
$\therefore $Secondary carbocation is more stable than primary carbocation due to$ + \,I$ effect of two methyl groups.
(ii) In the following step the nucleophile$\left( {B{r^ - }} \right)$ attacks the secondary carbocation to form secondary halide.
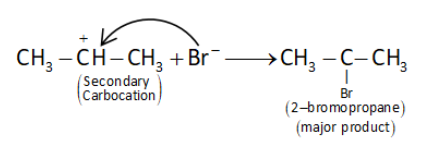
Hence, the correct answer is option b.
Note: In the presence of peroxide, addition of $HBr$to unsymmetrical alkene like propene takes place contrary to the Markonikov’s rule. This happens only with$HBr$but not with $HCl$or$HI$. This reaction is known as an additional reaction which is anti to Markonikov’s rule. Markonikov’s rule has no value in case of symmetrical alkenes because there is no identification of highly unsaturated carbon.
Complete step by step solution:
According to Markonikov’s rule: During unsymmetrical alkene, the negative part of the attacking reagent goes to the carbon carrying less number of hydrogen atoms and positive part goes to the other carbon atom. In other words the addition of hydrogen takes place in the highly unsaturated carbon atom of alkene.
For example: Addition of$HBr$to unsymmetrical alkene. When $HBr$is added to an alkene which is unsymmetrical, two types of products are formed as given below:

Mechanism: It is an electrophilic addition which is favoured by the formation of more stable carbonium ion intermediate.
(i) $HBr$undergoes ionisation in the presence of propene molecule to give electrophile $\left( {{H^ + }} \right)$and nucleophile $\left( {B{r^ - }} \right)$.
$H - Br\xrightarrow{{}}\mathop {{H^ \oplus }}\limits_{\left( {electrophile} \right)} + \mathop {B{r^ - }}\limits_{\left( {nucleophile} \right)} $
(ii) ${H^ \oplus }$attacks the double bond to produce a more stable secondary carbocation ion.

$\therefore $Secondary carbocation is more stable than primary carbocation due to$ + \,I$ effect of two methyl groups.
(ii) In the following step the nucleophile$\left( {B{r^ - }} \right)$ attacks the secondary carbocation to form secondary halide.

Hence, the correct answer is option b.
Note: In the presence of peroxide, addition of $HBr$to unsymmetrical alkene like propene takes place contrary to the Markonikov’s rule. This happens only with$HBr$but not with $HCl$or$HI$. This reaction is known as an additional reaction which is anti to Markonikov’s rule. Markonikov’s rule has no value in case of symmetrical alkenes because there is no identification of highly unsaturated carbon.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




