
A vicinal diol has two alcoholic groups present:
A.on the same carbon
B.on adjacent carbon
C.anywhere along with the carbon
D.none of the above
Answer
239.4k+ views
Hint: We know that there are two types of groups one is vicinal and one is a geminal diol. One group has two hydroxyl groups on the same carbon and the other has two hydroxyl groups on different carbon.
Complete step by step answer:
We know that the vicinal meaning is adjacent or neighboring.
Let us see the example of vicinal diol:
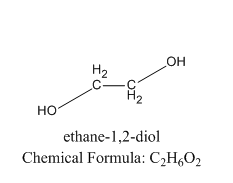
As we can see that the two alcoholic groups are present on the adjacent carbon in vicinal diol.
Therefore, we can conclude that the correct answer to this question is option B.
Additional Information:
Let us see the structure of geminal diol to understand the difference between vicinal alcohol and geminal alcohol.
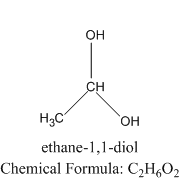
As we can see that the two alcoholic groups are present on the same carbon in geminal diol.
We know that in a vicinal diol, the two hydroxyl groups occupy neighboring positions, that is, they are attached to adjacent atoms and these compounds are called glycols. Examples include 1,2-ethanediol or ethylene glycol \[HO - {(C{H_2})_2} - OH\], a common ingredient of antifreeze products. Another example is propane-1,2-diol, or alpha propylene glycol, \[HO - C{H_2} - CH\left( {OH} \right) - C{H_3}\], used in the food and medicine industry, as well as a relatively non-poisonous antifreeze product.
We most know that on commercial scales, the main route to vicinal diols is the hydrolysis of epoxides and the epoxides are prepared by epoxidation of the alkene.
Note: We can get confused between the vicinal and geminal alcohol and we must know the placement of the alcoholic group on the adjacent carbon atom and same carbon atom respectively. So, option A and option C can be neglected.
Complete step by step answer:
We know that the vicinal meaning is adjacent or neighboring.
Let us see the example of vicinal diol:

As we can see that the two alcoholic groups are present on the adjacent carbon in vicinal diol.
Therefore, we can conclude that the correct answer to this question is option B.
Additional Information:
Let us see the structure of geminal diol to understand the difference between vicinal alcohol and geminal alcohol.

As we can see that the two alcoholic groups are present on the same carbon in geminal diol.
We know that in a vicinal diol, the two hydroxyl groups occupy neighboring positions, that is, they are attached to adjacent atoms and these compounds are called glycols. Examples include 1,2-ethanediol or ethylene glycol \[HO - {(C{H_2})_2} - OH\], a common ingredient of antifreeze products. Another example is propane-1,2-diol, or alpha propylene glycol, \[HO - C{H_2} - CH\left( {OH} \right) - C{H_3}\], used in the food and medicine industry, as well as a relatively non-poisonous antifreeze product.
We most know that on commercial scales, the main route to vicinal diols is the hydrolysis of epoxides and the epoxides are prepared by epoxidation of the alkene.
Note: We can get confused between the vicinal and geminal alcohol and we must know the placement of the alcoholic group on the adjacent carbon atom and same carbon atom respectively. So, option A and option C can be neglected.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
Difference Between Crystalline and Amorphous Solid

Understanding Electromagnetic Waves and Their Importance

Common Ion Effect: Concept, Applications, and Problem-Solving

Correct order of basic strength of given amines is class 11 chemistry JEE_Main

Understanding the Electric Field of a Charged Spherical Shell

Understanding How a Current Loop Acts as a Magnetic Dipole

Other Pages
Understanding the Electric Field Due to Infinite Linear Charge and Cylinders

JEE Main Colleges 2026: Complete List of Participating Institutes

Understanding Collisions: Types and Examples for Students

How Does Fusion Reaction Happen Inside the Sun?

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding EMF and Internal Resistance of a Cell




