
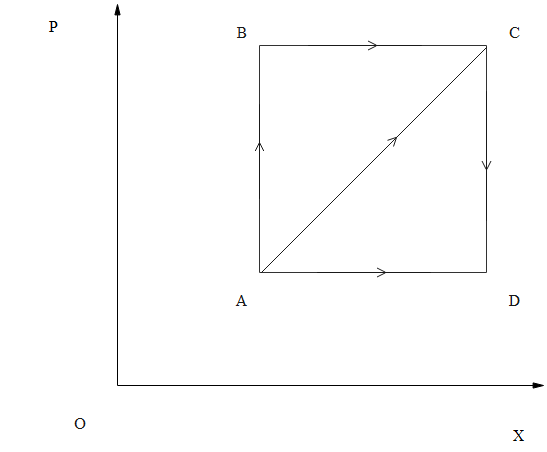
A thermodynamic process is shown in the figure. The pressure and volumes corresponding to some points in the figure are:
${{\text{P}}_{\text{A}}}=3\times {{10}^{4}}\text{Pa}$
$\mathrm{V}_{\mathrm{A}}=2 \times 10^{-3} \mathrm{m}^{-3}$
${{\text{P}}_{\text{B}}}=8\times {{10}^{4}}\text{Pa}$
$\mathrm{V}_{\mathrm{C}}=5 \times 10^{-3} \mathrm{m}^{-3}$
In the process A B, 600 J of heat is added to the system and in process B C, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be
(A) 560 J
(B) 800 J
(C) 600 J
(D) 640 J
Answer
232.8k+ views
Hint: We know that thermodynamics is the branch of physics that deals with the relationships between heat and other forms of energy. In particular, it describes how thermal energy is converted to and from other forms of energy and how it affects matter. Traditionally, thermodynamics has stated three fundamental laws: the first law, the second law, and the third law. A more fundamental statement was later labelled the 'zeroth law'. The third law of thermodynamics states that a system's entropy approaches a constant value as the temperature approaches absolute zero. Entropy, the measure of a system's thermal energy per unit temperature that is unavailable for doing useful work. Because work is obtained from ordered molecular motion, the amount of entropy is also a measure of the molecular disorder, or randomness, of a system.
Complete step by step answer
We know that in physics and chemistry, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be conserved over time. For instance, chemical energy is converted to kinetic energy when a stick of dynamite explodes. The principle of the conservation of energy refers to the idea that energy is not created or lost – it is only transformed from one form to another. In all changes some energy is always converted into forms (mainly low-grade heat) that cannot be used to make further changes. This law is very important because it is a very easy way to figure out important information about an object. For example, if you know an object's mass and initial height, you can find its initial potential energy, which is all the energy that it starts with.
No work is done during the isochoric process $\mathrm{A} \rightarrow \mathrm{B}$.
Work done during isobaric process $\mathrm{B} \rightarrow \mathrm{C}=\mathrm{P}_{\mathrm{B}}\left(\mathrm{V}_{\mathrm{C}}-\mathrm{V}_{\mathrm{B}}\right)$
$=\mathrm{P}_{\mathrm{B}}\left(\mathrm{V}_{\mathrm{D}}-\mathrm{V}_{\mathrm{A}}\right)=240 \mathrm{J}$
From conservation of energy, $\mathrm{Q}=\mathrm{U}+\mathrm{W}$
$\Rightarrow \text{U}=\text{Q}-\text{W}=(600+200)\text{J}-240\text{J}=560\text{J}$
Hence the correct answer is option A.
Note: We know that an isobaric process is one in which a gas does work at constant pressure, while an isochoric process is one in which volume is kept constant. Because volume is constant in an isochoric process, no work is done. Because the volume change is zero in this case, the work done is zero. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. In the process, a gas either expands or contracts to maintain constant pressure and hence the net amount of work is done by the system or on the system. An Isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
Complete step by step answer
We know that in physics and chemistry, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be conserved over time. For instance, chemical energy is converted to kinetic energy when a stick of dynamite explodes. The principle of the conservation of energy refers to the idea that energy is not created or lost – it is only transformed from one form to another. In all changes some energy is always converted into forms (mainly low-grade heat) that cannot be used to make further changes. This law is very important because it is a very easy way to figure out important information about an object. For example, if you know an object's mass and initial height, you can find its initial potential energy, which is all the energy that it starts with.
No work is done during the isochoric process $\mathrm{A} \rightarrow \mathrm{B}$.
Work done during isobaric process $\mathrm{B} \rightarrow \mathrm{C}=\mathrm{P}_{\mathrm{B}}\left(\mathrm{V}_{\mathrm{C}}-\mathrm{V}_{\mathrm{B}}\right)$
$=\mathrm{P}_{\mathrm{B}}\left(\mathrm{V}_{\mathrm{D}}-\mathrm{V}_{\mathrm{A}}\right)=240 \mathrm{J}$
From conservation of energy, $\mathrm{Q}=\mathrm{U}+\mathrm{W}$
$\Rightarrow \text{U}=\text{Q}-\text{W}=(600+200)\text{J}-240\text{J}=560\text{J}$
Hence the correct answer is option A.
Note: We know that an isobaric process is one in which a gas does work at constant pressure, while an isochoric process is one in which volume is kept constant. Because volume is constant in an isochoric process, no work is done. Because the volume change is zero in this case, the work done is zero. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. In the process, a gas either expands or contracts to maintain constant pressure and hence the net amount of work is done by the system or on the system. An Isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




