
A: Tetracyanomethane
B: Carbon dioxide
C: Benzene
D: 1,3-buta-di-ene
Ratio of $\sigma $ and $\pi $:
(A) A and B are equal
(B) C and D are equal
(C) same in A, B, C and D
(D) B and D are equal
Answer
233.1k+ views
Hint: One single bond has only one sigma bond $\left( \sigma \right)$ and no pi bond $\left( \pi \right)$. One double bond contributes to one sigma bond $\left( \sigma \right)$ and one pi-bond $\left( \pi \right)$. One triple bond has one sigma bond $\left( \sigma \right)$ and two pi-bond $\left( \pi \right)$. Describe the structure and find the number of single, double and triple bonds and calculate the ratios.
Complete step by step solution:
Let us draw the structures of the four structures given in the question:
The ratio of $\sigma $ and $\pi $ in A and B are equal, which is option (A).
Note: An element forms a sigma bond $\left( \sigma \right)$ with every different element only once. That is if an element forms multiple bonds with another element, then, it means that there is one sigma bond $\left( \sigma \right)$ and rest are pi-bonds. The compounds with single bonds are known as saturated and compounds with multiple bonds are unsaturated.
Complete step by step solution:
Let us draw the structures of the four structures given in the question:
| S. No. | Name of the compounds | Structure of the compounds | Explanation of the bonds and formation of the structure | Number of sigma bonds $\left( \sigma \right)$ | Number of pi-bonds $\left( \pi \right)$ | Ratio of $\sigma $ and $\pi $ |
| 1. | Tetracyanomethane | 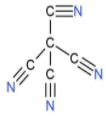 | The compound has four cyano groups. A carbon atom is attached to the carbon atom of a cyano group by a single bond. There is a triple bond between nitrogen and carbon atoms in the cyano group. So, there are four triple bonds. | $\left( 1\times 4 \right)+\left( 1\times 4 \right)$, which is equal to 8. | $\left( 2\times 4 \right)$, which is equal to 8. | $\dfrac{8}{8}$ or 1. |
| 2. | Carbon dioxide | 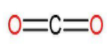 | The compound has a single carbon atom which forms a double bond with two oxygen atoms. | $\left( 1\times 2 \right)$ or 2 | $\left( 1\times 2 \right)$ or 2 | $\dfrac{2}{2}$ or 1 |
| 3. | Benzene | 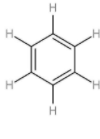 | The compound has six carbon atoms which form an alternative double bond with each other. Every carbon atom is attached to a hydrogen atom with a single bond. The chemical formula is ${{\text{C}}_{6}}{{\text{H}}_{6}}$. | $\left( 1\times 6 \right)+\left( 1\times 6 \right)$ or 12 | $\left( 1\times 3 \right)$ or 3 | $\dfrac{12}{3}$ or 4 |
| 4. | 1,3-buta-di-ene | 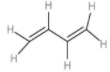 | The compound has an alternative double bond. The total number of hydrogen atoms is 6. | $\left( 1\times 6 \right)+\left( 1\times 3 \right)$or 9 | $\left( 1\times 2 \right)$ or 2 | $\dfrac{9}{2}$ or 4.5 |
The ratio of $\sigma $ and $\pi $ in A and B are equal, which is option (A).
Note: An element forms a sigma bond $\left( \sigma \right)$ with every different element only once. That is if an element forms multiple bonds with another element, then, it means that there is one sigma bond $\left( \sigma \right)$ and rest are pi-bonds. The compounds with single bonds are known as saturated and compounds with multiple bonds are unsaturated.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




