
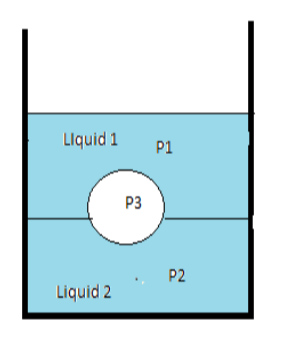
A jar filled with two non-mixing liquids 1 and 2 having densities ${\rho _1}$ and ${\rho _2}$, respectively. A solid ball, made of a material of density ${\rho _3}$, is dropped in the jar. It comes to equilibrium in the position shown in the figure. Which of the following is true ${\rho _{1,}}$ ${\rho _2}$ and ${\rho _3}$?
A) ${\rho _1} < {\rho _2} < {\rho _3}$
B) ${\rho _2} < {\rho _1} < {\rho _3}$
C) ${\rho _1} < {\rho _3} < {\rho _2}$
D) ${\rho _1} > {\rho _2} > {\rho _3}$
Answer
233.1k+ views
Hint: Density is a measure of mass per volume, the symbol most often used for density is $\rho $.
Then, formula used for density is:
$Density = \dfrac{{mass}}{{volume}}$
The more is mass, the more will be density.
Complete step by step solution:
Let us define density first and then we will determine the density of the liquids in increasing order. The density of a substance is its mass per unit volume. The density varies with temperature and pressure. On increasing the pressure on an object volume decreases and thus the density increases(as per the formula given in hint, density is inversely proportional to volume).
Density is an intensive property in that increasing the amount of as substance does not increase its density rather its mass increases.
When the object, substance or liquid has more mass then its density is also more.
As per the given diagram of the question, the liquid 2 which has more density will settle at the bottom then the object which is ball having density less than the liquid 2 will float on it. Similarly liquid 1 has density less than both the ball and liquid 2 will float on liquid 2 and the ball will sink in liquid 1.
Thus we can conclude that liquid 2 has maximum density, then ball and liquid 1.
Hence, option (C) is correct.
Note: Density of an object or substance determines whether or not an object will float on the surface of water . If the density of an object is lesser than the water than the object will float on the surface of water and if the density of an object is greater than the object will sink at the bottom of the water tank.
Then, formula used for density is:
$Density = \dfrac{{mass}}{{volume}}$
The more is mass, the more will be density.
Complete step by step solution:
Let us define density first and then we will determine the density of the liquids in increasing order. The density of a substance is its mass per unit volume. The density varies with temperature and pressure. On increasing the pressure on an object volume decreases and thus the density increases(as per the formula given in hint, density is inversely proportional to volume).
Density is an intensive property in that increasing the amount of as substance does not increase its density rather its mass increases.
When the object, substance or liquid has more mass then its density is also more.
As per the given diagram of the question, the liquid 2 which has more density will settle at the bottom then the object which is ball having density less than the liquid 2 will float on it. Similarly liquid 1 has density less than both the ball and liquid 2 will float on liquid 2 and the ball will sink in liquid 1.
Thus we can conclude that liquid 2 has maximum density, then ball and liquid 1.
Hence, option (C) is correct.
Note: Density of an object or substance determines whether or not an object will float on the surface of water . If the density of an object is lesser than the water than the object will float on the surface of water and if the density of an object is greater than the object will sink at the bottom of the water tank.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




