
A ${C}_{8}{H}_{10}$ hydrocarbon is nitrated by $HN{O}_{3}$ and sulphuric acid. Two, and only two, ${C}_{8}{H}_{9}N{O}_{2}$ isomers are obtained. Which of the following fits into the evidence?
a) Ethyl benzene
b) Ortho-xylene
c) Meta-xylene
d) Para-xylene
Answer
232.5k+ views
Hint: Xylene is a common name for dimethyl benzene. The two methyl groups could be attached at ortho, meta or para position of the benzene ring.
Complete step by step solution:
● In the reaction given, if the only a single alkyl group is attached to the benzene ring, ethyl group in this case, the products that would be formed on reacting with $HN{O}_{3}$ and sulphuric acid will definitely have 3 isomers as the nitro group will have three carbons that it can attach to in the benzene ring. Those positions are ortho, meta, and para positions. But it is given in the question that only two isomers are formed of the reactant.
● If the attached group would have been a larger group, then the ortho position would have been blocked and only two isomers would have been formed. But this is not the case in this situation.
● For the product to have only two isomers it is important to have two alkyl groups attached to the benzene ring. And in this case, those alkyl groups are two identical methyl groups.
● But now the main question arises in which position these methyl groups are to be attached, ortho, meta or para.
● If they are attached at meta positions then, three isomers of the compound is possible.
● If they are attached at para position only one isomer is possible.
● But if we attach them at ortho position only two isomers are possible.
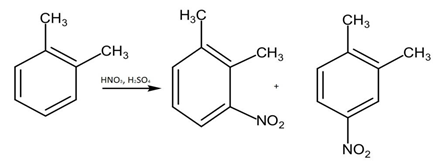
Hence, the ortho xylene will give us the desired product. Therefore, the correct answer is option (b).
Note: While making the isomers do make sure that you provide the numbering according to the standard rules and do not include the same isomers. If you don't follow the rules while making the isomers you might end up with more no. of isomers than expected.
Complete step by step solution:
● In the reaction given, if the only a single alkyl group is attached to the benzene ring, ethyl group in this case, the products that would be formed on reacting with $HN{O}_{3}$ and sulphuric acid will definitely have 3 isomers as the nitro group will have three carbons that it can attach to in the benzene ring. Those positions are ortho, meta, and para positions. But it is given in the question that only two isomers are formed of the reactant.
● If the attached group would have been a larger group, then the ortho position would have been blocked and only two isomers would have been formed. But this is not the case in this situation.
● For the product to have only two isomers it is important to have two alkyl groups attached to the benzene ring. And in this case, those alkyl groups are two identical methyl groups.
● But now the main question arises in which position these methyl groups are to be attached, ortho, meta or para.
● If they are attached at meta positions then, three isomers of the compound is possible.
● If they are attached at para position only one isomer is possible.
● But if we attach them at ortho position only two isomers are possible.

Hence, the ortho xylene will give us the desired product. Therefore, the correct answer is option (b).
Note: While making the isomers do make sure that you provide the numbering according to the standard rules and do not include the same isomers. If you don't follow the rules while making the isomers you might end up with more no. of isomers than expected.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main 2026 Answer Key OUT Check PDF Response Sheet

JEE Main 2026 Admit Card OUT LIVE | Session 1 Direct Download Link

JEE Main 2023 April 8 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 8 Shift 2 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

JEE Mains 2026 January 21 Shift 2 Question Paper with Solutions PDF - Complete Exam Analysis

JEE Main 2026 Jan 22 Shift 2 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

List of Highest T20 Scores in International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




