
(A)
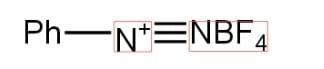
(B)
(C)
(D)
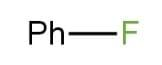
Answer
232.8k+ views
Hint: Aniline is an organic compound in which $N{{H}_{2}}$ group is attached to a benzene ring. It is also known as phenylamine. This compound gets dark when exposed to air and light. It is a highly toxic compound. The behaviour of aniline is somewhat similar to that of primary aliphatic amines. It boils at a temperature of ${{183}^{o}}C$.
Complete Step by Step Answer:
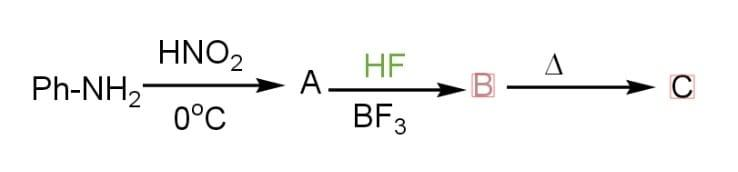
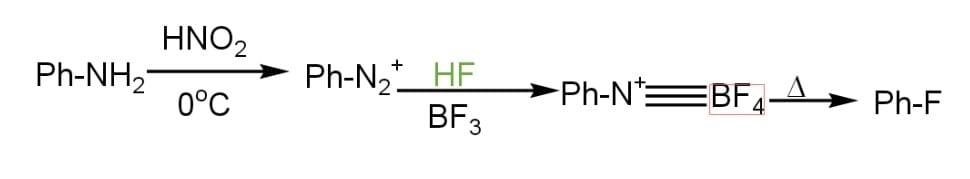
Aniline and $HN{{O}_{2}}$ combine in the first step to produce diazonium salt, which is then combined with hydrogen fluoride ($HF$) and fluoroboric acid ($B{{F}_{3}}$) to create fluoro benzene ($PhF$). Fluorobenzene is a colourless liquid having the molecular formula ${{C}_{6}}{{H}_{5}}F$. It is also known as phenyl fluoride. It is a flammable substance.
Correct Option: (D) $Ph-F$.
Additional Information: The reagent, nitrous acid ($HN{{O}_{2}}$), which is used to convert aniline to diazonium salt, is prepared by the reaction of sodium nitrite ($NaN{{O}_{2}}$) and hydrochloric acid ($HCl$). The diazonium salt formed is unstable at room temperature and is usually prepared at a very low temperature (${{0}^{o}}-{{5}^{o}}C$). Diazonium salts, in general, are high-energy substances that can violently break down when heated or forced mechanically (shock-sensitive). It is an important intermediate for producing substituted benzene.
Note: Aniline is a highly used compound in the clothing industry. It is used as a dyeing agent. For the manufacturing of rubber chemicals and goods like automobile tyres, balloons, gloves, etc., anilines are employed in the rubber industry. Insecticide and reagents for plastic and resin polymers are uses of fluorobenzene.
Complete Step by Step Answer:
Aniline and $HN{{O}_{2}}$ combine in the first step to produce diazonium salt, which is then combined with hydrogen fluoride ($HF$) and fluoroboric acid ($B{{F}_{3}}$) to create fluoro benzene ($PhF$). Fluorobenzene is a colourless liquid having the molecular formula ${{C}_{6}}{{H}_{5}}F$. It is also known as phenyl fluoride. It is a flammable substance.

Correct Option: (D) $Ph-F$.
Additional Information: The reagent, nitrous acid ($HN{{O}_{2}}$), which is used to convert aniline to diazonium salt, is prepared by the reaction of sodium nitrite ($NaN{{O}_{2}}$) and hydrochloric acid ($HCl$). The diazonium salt formed is unstable at room temperature and is usually prepared at a very low temperature (${{0}^{o}}-{{5}^{o}}C$). Diazonium salts, in general, are high-energy substances that can violently break down when heated or forced mechanically (shock-sensitive). It is an important intermediate for producing substituted benzene.
Note: Aniline is a highly used compound in the clothing industry. It is used as a dyeing agent. For the manufacturing of rubber chemicals and goods like automobile tyres, balloons, gloves, etc., anilines are employed in the rubber industry. Insecticide and reagents for plastic and resin polymers are uses of fluorobenzene.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




