
\[1,2\;\]di-bromocyclohexane on dehydrohalogenation gives
A. 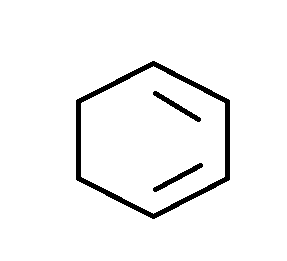
B. 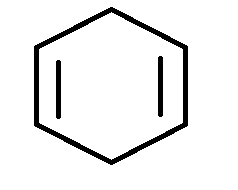
C. 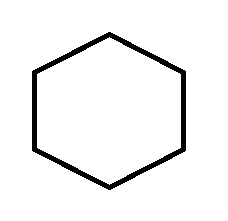
D. 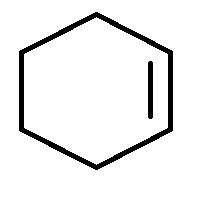
Answer
241.2k+ views
Hint: \[1,2\;\]di-bromocyclohexane has the following structure
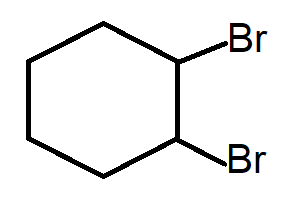
Dehydrohalogenation is a chemical reaction that undergoes removal of halogen and hydrogen to form alkene. ‘de’ means removal, ‘hydro’ means hydrogen, ‘halogen’ means halogen. The halogen and hydrogen must be present on adjacent positions.
Complete step-by-step answer:Dehydrohalogenation takes place in presence of a strong base like $KOH$. The $O{H^ - }$ accepts a hydrogen from the adjacent position of a good leaving group resulting in formation of positive charge which converts into a double bond by removal of the leaving group, namely halide for this particular reaction. In reality, the positive charge is actually not formed because the entire process occurs in a concerted manner. It is only to understand the basic mechanism. The elimination follows $E2$ mechanism which is a one step process with no intermediate (or positive charge) but only proceeds through the transition state. In \[1,2\;\] di-bromocyclohexane , the two bromides are present at \[1,2\;\] positions on cyclohexane. Each bromide will leave along with adjacent hydrogen to form a product similar to option A.
Hence A is the correct answer.
Option ‘A’ is correct
Additional Information: Alkene formation can follow Saytzeff elimination (more substituted alkene) or Hofmann elimination (less substituted alkene). Saytzeff elimination is followed in case of neutral molecules and Hofmann in case of charged species.
Note: Dehydrohalogenation requires proper stereo or proper orientation of the atoms. To undergo elimination, halogen and hydrogen must be anti-planner to each other. Hence, dehydrohalogenation is a stereospecific reaction. Dehalogenation reaction is also a type of reaction which eliminates two halogens present at \[1,2\;\]. But it is carried out only in presence of reagents like zinc dust,$NaI$, $MeOH$OR $EtOH$.

Dehydrohalogenation is a chemical reaction that undergoes removal of halogen and hydrogen to form alkene. ‘de’ means removal, ‘hydro’ means hydrogen, ‘halogen’ means halogen. The halogen and hydrogen must be present on adjacent positions.
Complete step-by-step answer:Dehydrohalogenation takes place in presence of a strong base like $KOH$. The $O{H^ - }$ accepts a hydrogen from the adjacent position of a good leaving group resulting in formation of positive charge which converts into a double bond by removal of the leaving group, namely halide for this particular reaction. In reality, the positive charge is actually not formed because the entire process occurs in a concerted manner. It is only to understand the basic mechanism. The elimination follows $E2$ mechanism which is a one step process with no intermediate (or positive charge) but only proceeds through the transition state. In \[1,2\;\] di-bromocyclohexane , the two bromides are present at \[1,2\;\] positions on cyclohexane. Each bromide will leave along with adjacent hydrogen to form a product similar to option A.
Hence A is the correct answer.
Option ‘A’ is correct
Additional Information: Alkene formation can follow Saytzeff elimination (more substituted alkene) or Hofmann elimination (less substituted alkene). Saytzeff elimination is followed in case of neutral molecules and Hofmann in case of charged species.
Note: Dehydrohalogenation requires proper stereo or proper orientation of the atoms. To undergo elimination, halogen and hydrogen must be anti-planner to each other. Hence, dehydrohalogenation is a stereospecific reaction. Dehalogenation reaction is also a type of reaction which eliminates two halogens present at \[1,2\;\]. But it is carried out only in presence of reagents like zinc dust,$NaI$, $MeOH$OR $EtOH$.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




