




Introduction to Energetics or Chemical Energetics
Let's see what happens if we pour a drop of water into an uncovered petri dish. The water evaporates after a few minutes. Let's try a different experiment in a covered petri dish. What is going to happen? After the same time interval as the first experiment, it remains on the plate.
We can ask a number of questions about these experiments. What causes these events? How quickly do they occur? Is it possible to provide a chemical explanation for these events? What is the name of this procedure? What is the name of the reversal procedure? Where have you seen it in your day-to-day life? What other effects did the initial process have? Thermochemistry, kinetics, and thermodynamics are used to study all these topics. As a result, chemical energetics plays a significant role in advanced physical chemistry.
Important Topics for Chemical Energetics
Enthalpy Change
Hess Law
Born - Haber Cycle
Bond Energies
Reaction Pathway
Entropy Change
Gibbs Free Energy
Spontaneity
Glycolysis
Important Concepts for Chemical Energetics
Enthalpy change, ΔH
The thermal energy held in a chemical system is known as enthalpy. It can't be directly measured.
Energy changes occur as a result of chemical processes (typically heat energy changes).
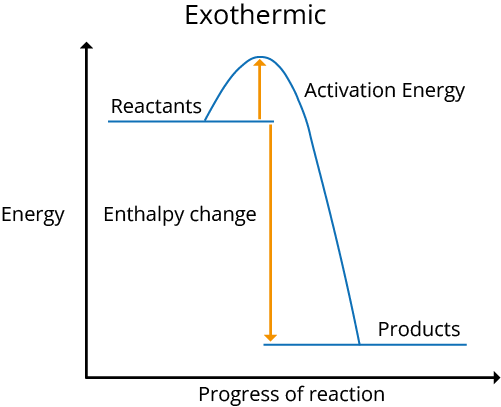
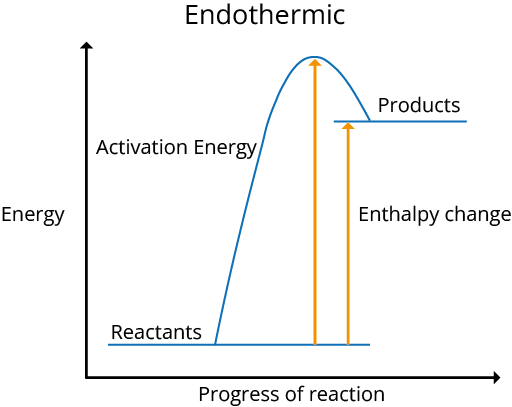
The reaction is exothermic when energy is discharged into the environment, and H is negative. The reaction is endothermic and H is positive if energy is taken in from the environment.
During an exothermic reaction, the temperature of the environment rises, while during an endothermic reaction, it falls.
The energy required to break one mole of a particular gaseous bond and produce atoms is referred to as bond energy.
As the actual enthalpy of a bond changes depending on which molecule it is in, bond enthalpies are given as a mean average in the data book.
Breaking bonds requires energy, hence ΔH is positive (endothermic). When bonds are formed, energy is released, hence ΔH is negative (exothermic).
The equation below can be used to calculate the enthalpy change of a reaction:
H = -mcΔT
where, H stands for enthalpy change (J)
m is the mass of the environment (g)
c stands for specific heat capacity (J g-1 K-1)
ΔT - temperature change (in kelvin or degrees Celsius)
Standard terms used in Enthalpy
Lattice Energy
The enthalpy change that occurs when one mole of a substance is produced from its gaseous ions is known as lattice formation enthalpy.
The enthalpy shift that occurs when one mole of an ionic substance is broken down to form its gaseous ions is known as lattice dissociation enthalpy. ΔH is negative when gaseous ions are converted into a solid lattice.
Lattice enthalpy is influenced by two factors:
Ionic charge: Increased ionic charge increases the attraction between positive and negative ions, resulting in a greater and more negative lattice formation enthalpy.
Ionic radius: A smaller ionic radius means the ions are closer together in the lattice, resulting in a greater attraction between them, resulting in a bigger, more negative lattice formation enthalpy.
Hess’ Law
According to Hess' Law, the enthalpy shift that occurs as a result of a chemical transition is unaffected by the route it follows. This is due to the fact that the reactants and products have the same enthalpy.
Any energy released during the intermediate formation process will be used to break bonds, allowing the product to form. The enthalpy change of route 1 is the same as route 2 in the diagram below:
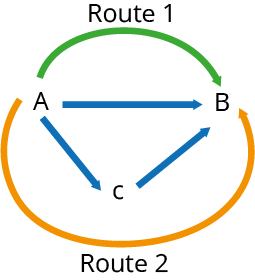
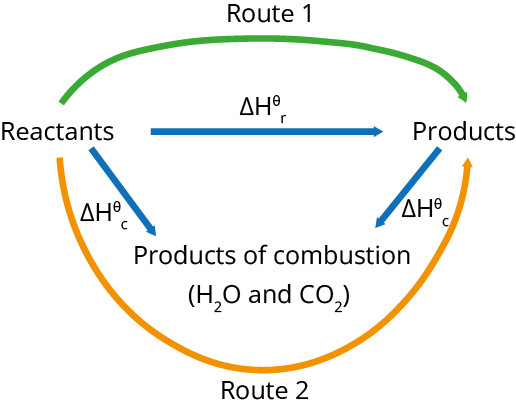
Hess' Law can be combined with the enthalpy change of production and combustion to determine the enthalpy change of a reaction.
It's crucial to pay attention to the directions of the enthalpy change arrows in the following cycles:
To find the enthalpy change of a reaction, using the enthalpy change of combustion:
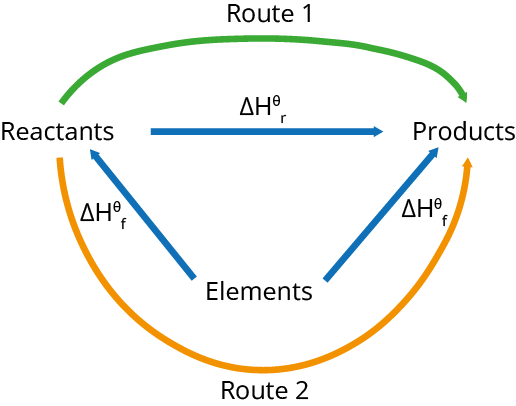
To find the enthalpy change of reaction, using the enthalpy change of formation:
Average Bond Energies
When all reactants and products are gaseous, bond energies can be utilised to calculate the enthalpy change of a process.
Method 1: ΔH = total energy required to break bonds minus total energy generated during bond formation. The average bond enthalpies for each bond must be multiplied by the number of that bond included in the equation when totaling the energy released or created.
The second method is to use an enthalpy cycle.
Example: CO(g) + H2O(g) → CO2(g) + H2(g)
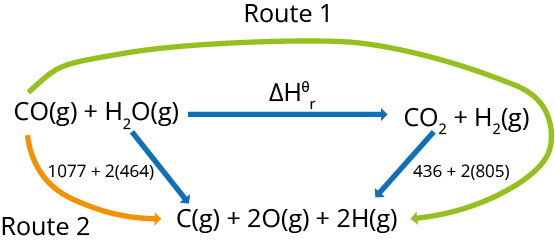
Using the diagram and following:
ΔH + 436 + 2(805) = 1077 + 2 (464)
ΔH = 1077 + 2(464) - 436 - 2H = 1077 + 2(464) - 436 - 2H = 1077 + 2(464) - (805)
ΔH = -41 kJ mol-1.
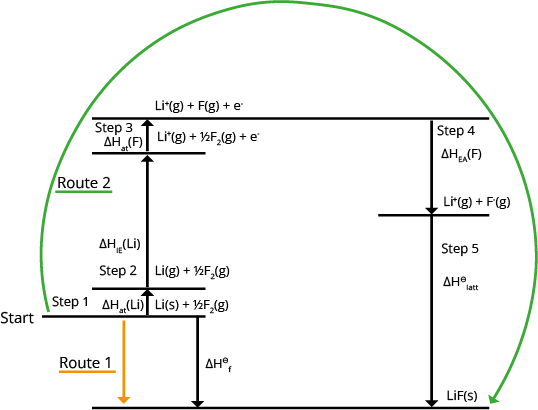
Born-Haber Cycles
Born-Haber cycles are used to calculate lattice enthalpy. The following enthalpy changes are used in Born-Haber cycles:
Enthalpy of formation on the lattice or enthalpy of dissociation on the lattice
Atomisation's enthalpy shift
Formation of enthalpy change
The energy necessary to remove one electron from one mole of gaseous atoms in order to generate one mole of gaseous 1+ ions is known as the first ionisation energy. This is only used for metals in Born-Haber cycles.
The energy produced when each atom in one mole of gaseous atoms receives an electron, generating one mole of gaseous 1-ions. This only applies to non-metals in Born-Haber cycles.
A Born-Haber cycle for lithium fluoride and the computation of the lattice enthalpy (ΔHӨLE) are shown below:
Reaction Pathway Diagrams
The following are diagrams of exothermic and endothermic reaction pathways:
Entropy change, ΔS
The degree of disorder in a system is measured by entropy. When there is more chaos and energy is spread out, a system becomes more stable.
From solid to liquid to gas, entropy increases, and watery substances have higher entropy than solids. This is because the energy spreads out as the particles get more disordered as their states change.
As gases have significantly higher entropy than liquids or solids due to their disordered mobility, the entropy change will be positive (entropy increases) if the number of gaseous moles increases throughout a process.
Increasing the temperature may create a state shift, increasing the entropy.
Entropy increases when the temperature rises without a change in the state because the particles have more kinetic energy and are more disordered as they move faster.
The following equation can be used to calculate the change in entropy of a reaction:
ΔS = ΣSϴ(products) - ΣSϴ(reactants)
Where Σ means ‘the sum of’ and the standard entropies of the reactants and products have been given.
A negative result indicates a drop in entropy, whereas a positive result suggests an increase in entropy.
Gibbs Free Energy Change, ΔG
This equation can be used to compute Gibbs free energy change:
ΔG = ΔH - TΔS; where
ΔG - Gibbs free energy change (kJ mol-1)
ΔH - enthalpy change (kJ mol-1)
T - temperature (K)
ΔS - entropy change (kJ K-1 mol-1)
When ΔG is less than or equal to 0, a response or process is spontaneous/possible.
If ΔG is greater than zero, the reaction is not spontaneous at the calculated temperature.
When you have H and S, rewrite the equation as ΔH - TΔS 0 and rearrange it to find the minimal temperature at which a reaction is spontaneous.
Spontaneity
When ΔH is negative and ΔS is positive: TΔS is negative since H is negative and TΔS is positive in the equation. As ΔG is always negative regardless of temperature, the reaction is spontaneous at all temperatures.
When ΔH is positive and ΔS is negative: the equation says that -TΔS is positive because ΔH is positive and TΔS is negative. ΔG is always positive and the reaction is never spontaneous because both terms are positive regardless of temperature.
When ΔH and ΔS are both positive: Because ΔH is positive and TΔS is positive in the equation, -TΔS is negative. As the temperature rises, -TΔS becomes increasingly negative. -TΔS will overwhelm H at high temperatures, and ΔG will be less than 0, resulting in a spontaneous reaction. At low temperatures, the reaction will not be spontaneous.
When ΔH and ΔS are both negative: TΔS is positive since ΔH is negative and TΔS is negative. At high temperatures, -TΔS becomes increasingly positive and outweighs ΔH, indicating that the reaction is not spontaneous. At low temperatures, the reaction will occur spontaneously.
Energetics of Glycolysis
Glycolysis is the process of breaking down glucose to produce energy. It generates two pyruvate molecules, ATP, NADH, and water.
The process occurs in a cell's cytoplasm and does not require oxygen.
It can be found in both aerobic and anaerobic bacteria.
The energetics of glycolysis include the formation of two molecules of glyceraldehyde 3-phosphate from one glucose molecule in the second stage of glycolysis, and the formation of two molecules of pyruvate as end products of glycolysis.
As a result, two molecules of glyceraldehyde 3-phosphate are used to calculate glycolysis energy.
Solved Examples from The Chapter
Question 1: Calculate the enthalpy change of combustion for a reaction in which 0.65 g of propan-1-ol was totally combusted and 150 g of water was heated from 20.1 to 45.5oC.
Solution:
Calculate the amount of energy that was utilised to heat the water.
Q = m x cp x ΔT,
∴ Q = 150 x 4.18 x 25.4,
∴ Q = 15925.8 J
Calculate how many moles of alcohol were combusted.
moles of propan-1-ol = mass/ Mr (molecular weight) = 0.65 / 60 = 0.01083 molΔcH is the enthalpy change per mole that is calculated (the enthalpy change of combustion).
ΔH = Q/ no of moles
∴ ΔH = 15925.8/0.01083
∴ ΔH = 1470073 J mol-1 = 1470 kJ mol-1 to 3 sf
Finally, add the sign to represent the energy change: if the temperature rises, the reaction is exothermic and is denoted by a negative sign, such as –1470 kJ mol-1.
Question 2: Calculate the temperature range in which this reaction can occur.
N2(g) + O2(g) → 2NO(g).
Solution:
∆ H = 180 kJ mol-1 ∆S = 25 J K-1 mol-1 The reaction will be feasible when ∆ G ≤0
Make ∆G = 0 in the following equation: ∆G = ∆H - T∆S
∴ 0 = ∆H - T∆S
So, T = ∆H / ∆S
T = 180/ (25/1000)
= 7200K
The T must be greater than 7200K, which is a very high temperature.
Solved Examples from Previous Year Question Papers
Question 1: When an ideal diatomic gas is heated at constant pressure the fraction of the heat energy supplied which increases the internal energy of the gas is:
(a) 2/5
(b) 3/5
(c) 3/7
(d) 5/7
Solution:
According to the first law of thermodynamics,
ΔU = q + WWhen under constant pressure,
ΔU = qp - PΔV
qp = ΔU + PΔV
Heat Energy Fraction that Increases Internal Energy
$= \frac{\bigtriangleup U}{q_p} \ = \frac{nC_v \bigtriangleup T}{nC_p \bigtriangleup T} \ = \frac{C_v}{C_p}$In the case of a diatomic gas,
Cp = 7R/2, & Cv = 5R/2.
Cv/Cp = 5/7.As a result, option (c) is the correct answer.
Question 2: Enthalpy change for a reaction depends upon
(a) physical state of reactants and products
(b) initial and final concentration
(c) number of steps in the reaction
(d) conditions of reaction
Solution:
ΔH of a reaction is determined by the following conditions:
(i) Reactants and products' physical states
(ii) Concentration at the start and end
(iii) The circumstances in which the reaction took place
As a result, option (a), (b), (d) is the correct answer.
Question 3: The equilibrium constant for the reaction, 2NOCl(g) → 2NO(g) + Cl2(g) at 400 K is . (Given ∆Ho = 77.2 kJ mol-1; ∆So = 122 J K-1 mol-1 (log1.95 = 0.292)
(a) Kc = 1.995 x 104
(b) Kc = 1.995 x 10-3
(c) Kc = 1.995 x 10-4
(d) Kc = 1.995 x 10-4
Solution:
∆Go = ∆H - T∆So
∆Go = 77.2 x 1000 - 400 x 22
∆Go = 77200 - 48800
∆Go = 28400 Jmol
As ∆Go = -2.303RT logKeq
Therefore, ∆Go = 1.95 x 10-5
As a result, option (d) is the correct answer.
Practice Questions
Question 1: What is the temperature at which methane melts?
CH4(s) → CH4(l) ∆H = 0.94 kJmol-1, ∆S = 10.3 Jmol-1 K
(a) T = 91 K
(b) T = 87 K
(a) T = 45 K
(a) T = 95 K
Answer: (a) T = 91 K
Question 2: Calculate the enthalpy of solution of NaCl if the lattice enthalpy of production of NaCl is -771 kJmol-1 and the hydration enthalpies of sodium and chloride ions are -406 kJmol-1 and -364 kJmol-1, respectively.
(a) -1 kJ mol-1
(b) +3 kJ mol-1
(c) +1 kJ mol-1
(d) -2 kJ mol-1
Answer: (c) +1 kJ mol-1
Conclusion of JEE Advanced Physical Chemistry Energetics Notes
Heat is absorbed or released in conjunction with all chemical processes. From the beginning, the intimate relationship between matter and energy has piqued people's interest and sparked discussion; it's no coincidence that fire was regarded as one of the four basic elements (together with earth, air, and water) as early as the fifth century BCE. Here we went over some of the essential ideas of energy and heat, as well as the relationship between them, in this unit.
FAQs on JEE Important Chapter - Energetics
1. In chemistry, what is energetics?
Exothermic processes in solution release energy and raise the temperature, whereas endothermic reactions absorb energy and lower the temperature. In reactions, bonds are broken and formed. When a chemical reaction occurs, energy is transferred to or from the surroundings. This frequently results in temperature variations that may be observed with a thermometer. Exothermic and endothermic reactions are also possible. Energetics is the study of the energy changes that occur in chemical reactions, whether exothermic or endothermic.
2. Is thermodynamics and energetics the same thing?
The distinction between thermodynamics and energetics is that energetics is a field of physics that investigates the flow and transformation of energy, whereas thermodynamics is the science of energy transformations.
3. What is the fundamental principle of chemistry and energetics?
By releasing or absorbing heat, all chemical reactions exchange energy with their surroundings. Energy is exchanged as pressure-volume work in reactions that produce or consume gases. Exothermic means that a chemical process produces heat. It is endothermic when a chemical reaction absorbs heat.























