




Introduction to Phenols
Phenol is a well-known word in the realm of organic chemistry. The chemical formula for phenol is C6H6O. C6H6O is an aromatic chemical with 4n+2 double bonds and a ring structure. A hydroxyl group coupled to a phenyl group may be seen in this molecule. It has been used as a cleaning agent since it is water soluble. Phenol was one of the first organic compounds to be used in the manufacture of industrial soap. In water, phenol is somewhat acidic and can erode human components such as the eyes, respiratory tract, and skin. Phenol is no longer utilised for cleaning as a result of these issues.
We will study numerous facts about phenols in this part.
Phenols are an important part of the JEE syllabus. We've covered all of the main aspects of Phenols in this article. This extensive Phenols article will help you gain an edge over your competitors.
Important Topics of Phenols
Methods of Preparation of Phenols
Classification of Phenol
Acidity of Phenols
What are Phenols?
Phenols are organic molecules with a benzene ring and a hydroxyl group attached. Carbolic acids are another name for them. Phenols create phenoxide when they react with active metals like sodium and potassium. The acidic character of phenol is demonstrated by its reactivity with metals.
In the year 1834, a scientist called Friedlieb Ferdinand Runge discovered phenol. He was able to get it out of coal tar. It's a crystalline solid that's white in colour. Because phenol may cause chemical burns to the skin, it must be handled with caution. Also, Phenolic acid is another name for this substance. Look for a six-membered aromatic ring that is immediately connected to a hydroxyl group to identify a member of this species.
Preparation of Phenols
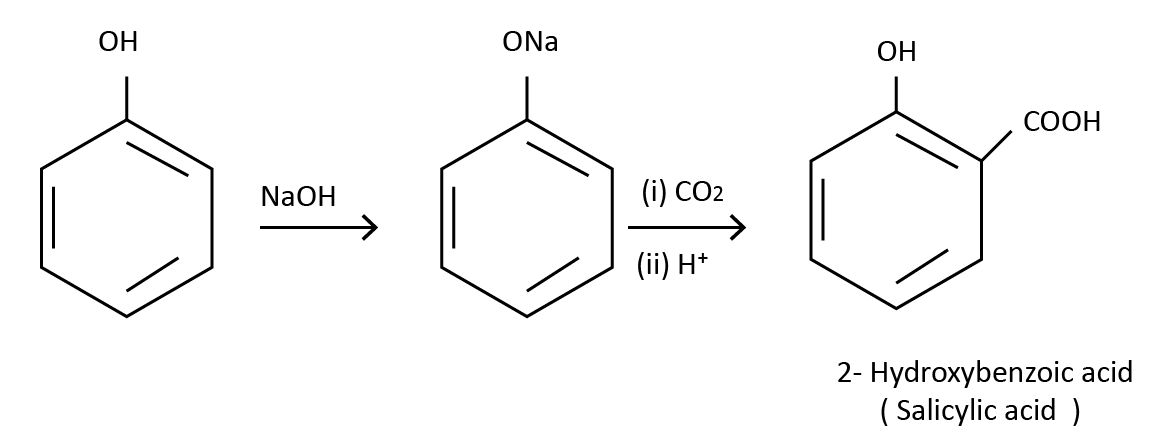
(1) From Benzene Sulphonic Acid: The sodium salt of benzene sulphonic acid is fused with sodium hydroxide to generate sodium phenoxide. When sodium phenoxide is exposed to dilute acid or carbon dioxide, it is transformed into phenol. This is one of the phenol preparation procedures used in laboratories.

(2) From Diazonium Salts: This is one of the most practical techniques for producing phenols. With the development of nitrogen gas, phenol is generated when benzene diazonium chloride solution is warmed with water or dilute acids.

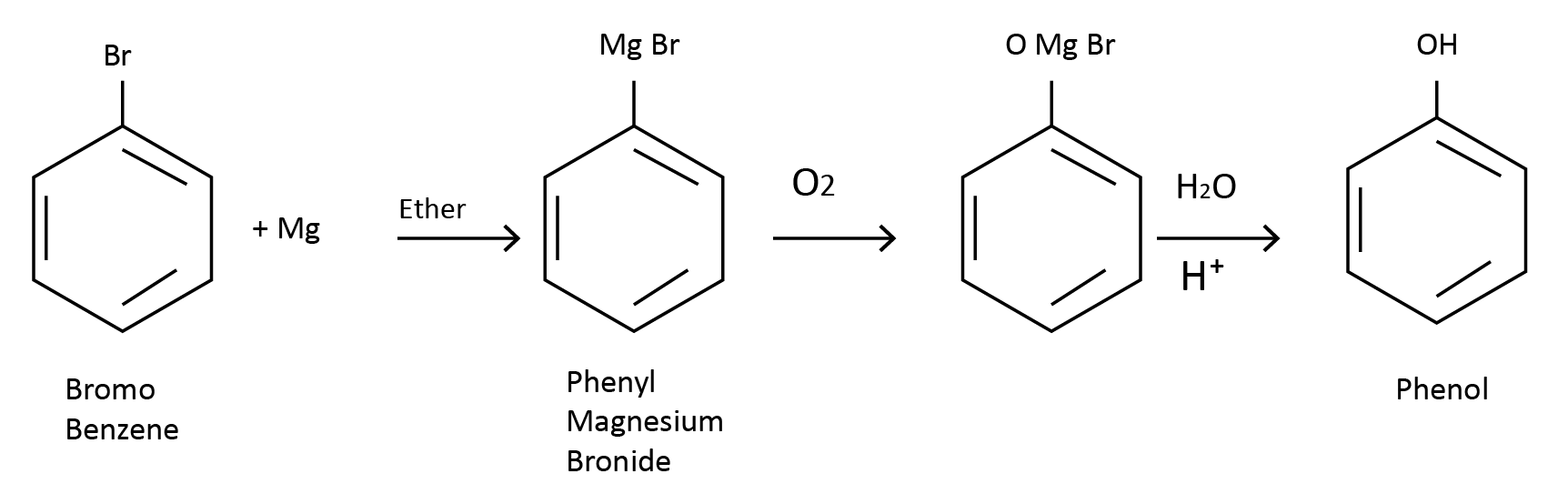
(3) From Grignard Reagent: Chlorobenzene or bromobenzene is first converted to phenyl magnesium halide in the presence of dry ether. When the Grignard reagent combines with oxygen, it forms phenol, which is subsequently hydrolyzed by a mineral acid.
(4) By Decarboxylation of Salicylic Acid: To make phenol, the sodium salt of salicylic acid is decarboxylated with soda lime and then acidified with weak hydrochloric acid.

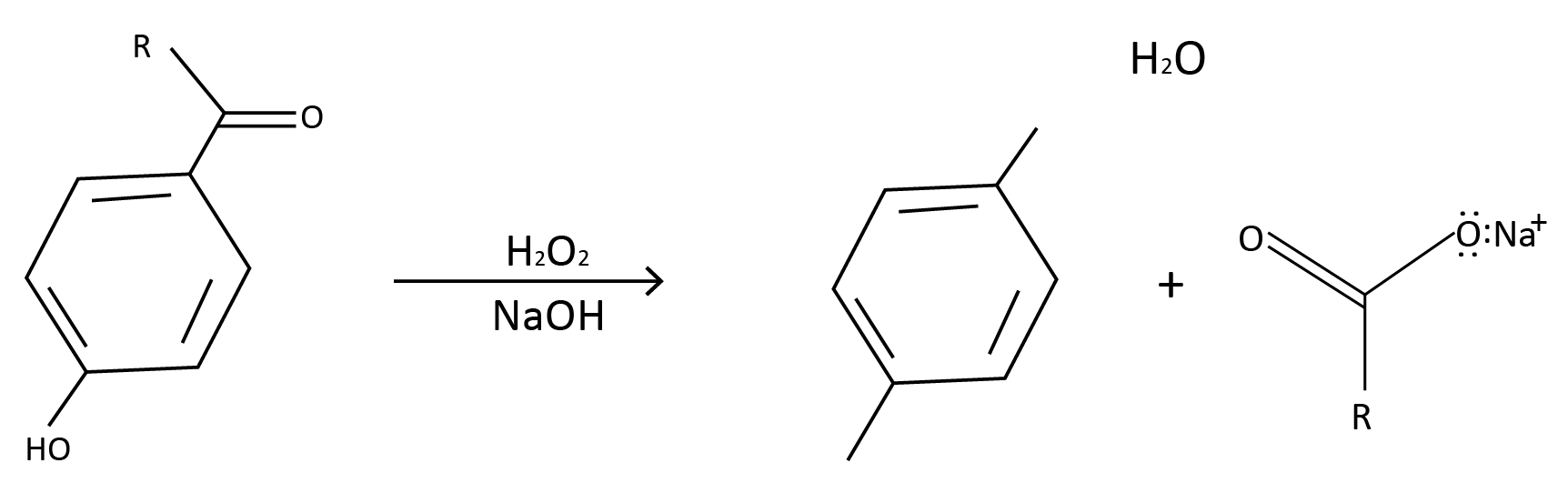
(5) From Dakin Reaction: When o– and p– hydroxy aldehydes or ketones (or o– and p– hydroxy amino aldehydes) react with alkaline hydrogen peroxide, they form o– and p– hydroxyphenol (or o– and p– aminophenol), which is then hydrolyzed to produce o– and p– aminophenol.
(6) Dow Process: In an alkaline atmosphere, chlorobenzene is hydrolysed. To make significant amounts of phenol, chlorobenzene is heated with a 10% solution of caustic soda or sodium carbonate at 300oC under high pressure.

(7) Raschig’s Process: Chlorobenzene is produced through the reaction of benzene, hydrogen chloride, and air at 523K in the presence of catalysts cupric chloride and ferric chloride. It is hydrolysed by superheated steam at 698K, resulting in phenol and HCl. This is a very new method of phenol synthesis. The HCl created can be used to convert more benzene to chlorobenzene.

Physical Properties of Phenol
The following are the physical properties of phenol:
Phenol is a deliquescent, colourless crystalline solid. When exposed to air and light, it becomes pink.
They may create intermolecular H-bonds with each other as well as with water. As a result, they have high boiling temperatures and are water-soluble.
It has a distinctive odour and a severe corrosive effect on the skin.
It is water-insoluble but readily soluble in organic solvents including alcohol, benzene, and ether.
Although toxic by nature, it has antiseptic and disinfectant properties.
Chemical Properties of Phenols
The chemical characteristics of phenol will be discussed in this section. We'll go over specific phenol reactions.
Electrophilic Substitution Reactions: The OH group on benzene raises the electron density on the benzene ring, making it more vulnerable to electrophile assault. Electrophilic substitution reactions are those that include the benzene ring. Because of the OH group, the ortho and para carbons of benzene have more electrons than the meta position. The OH group is also known as the o, p directing group.

Kolbe’s Reaction:
Reimer-Tiemann reaction:

Fries Rearrangement:

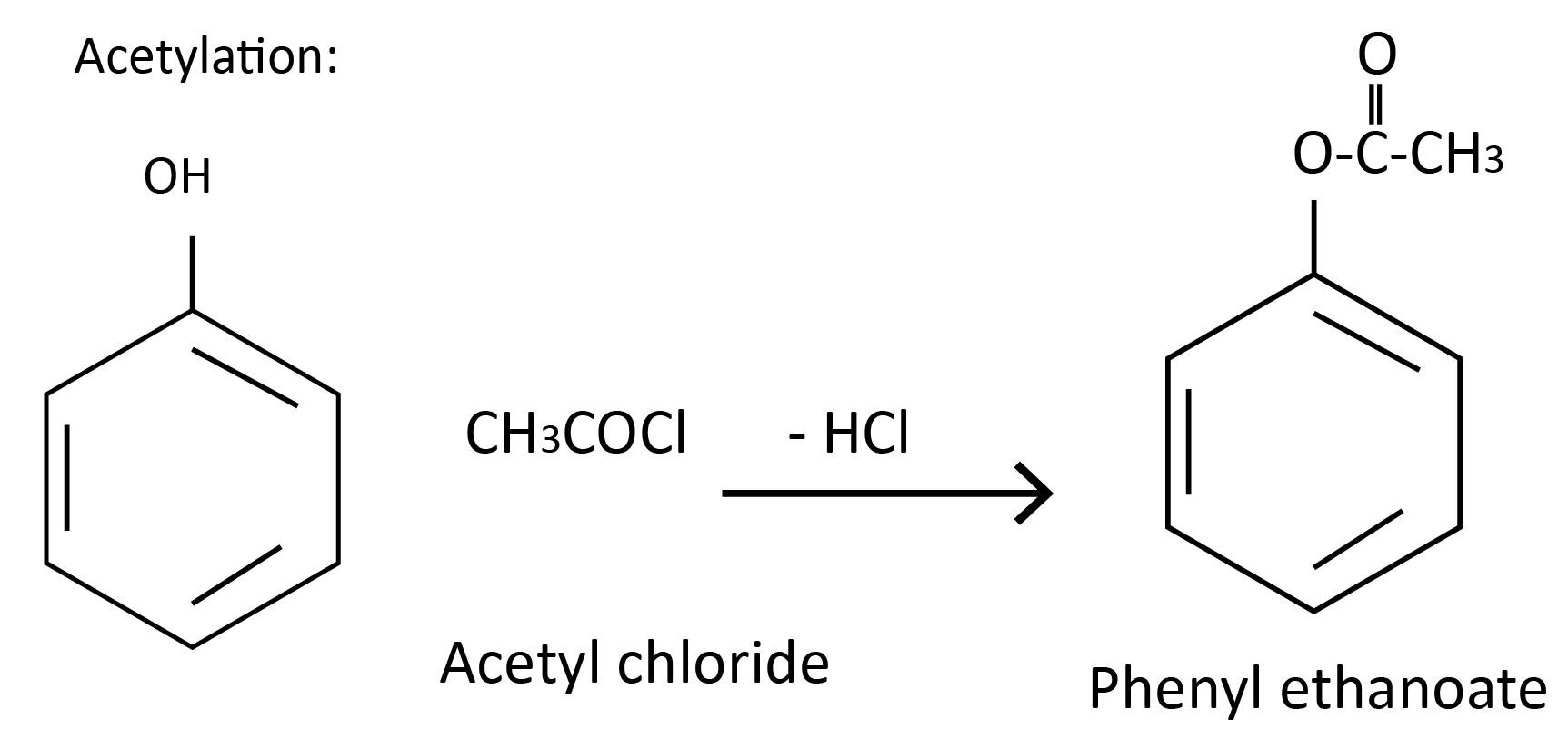
Acetylation:
Nitration:
Reaction with Dil. HNO3 :
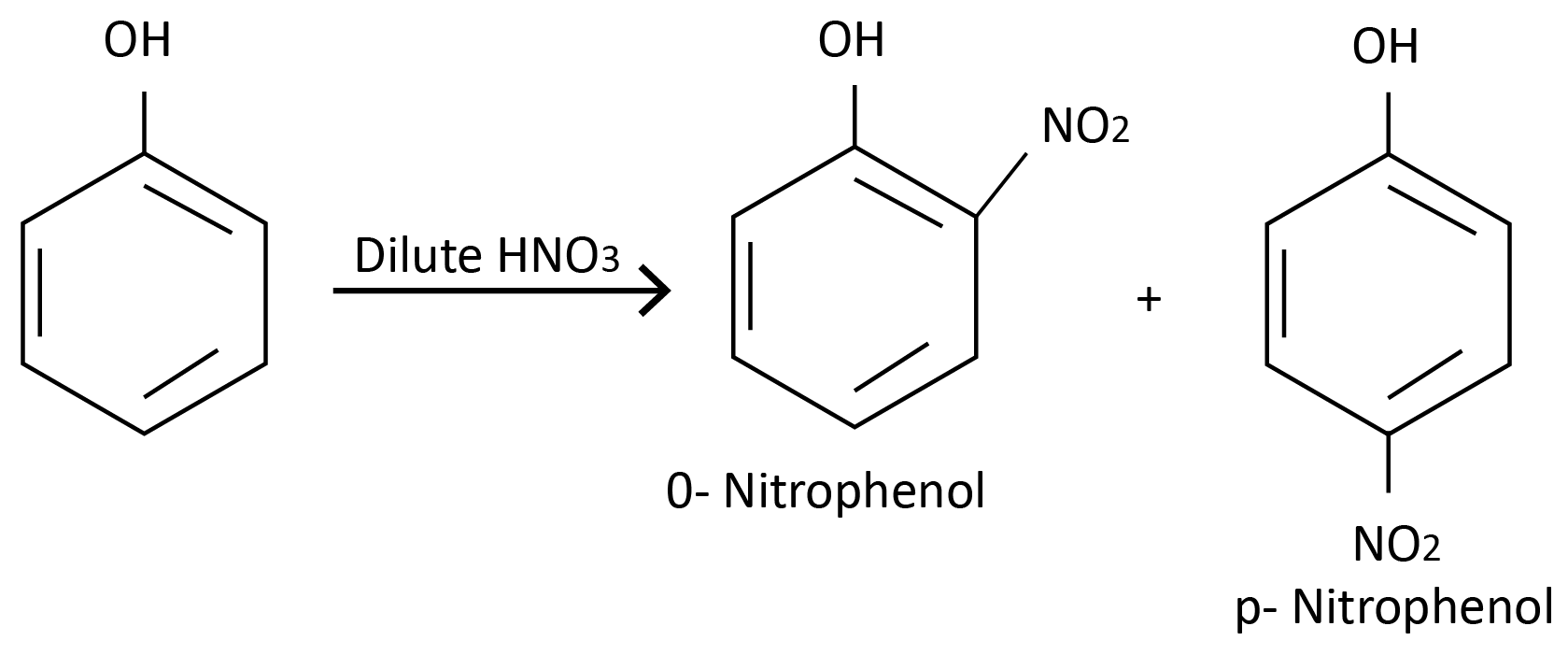
Reaction with Conc. HNO3 :
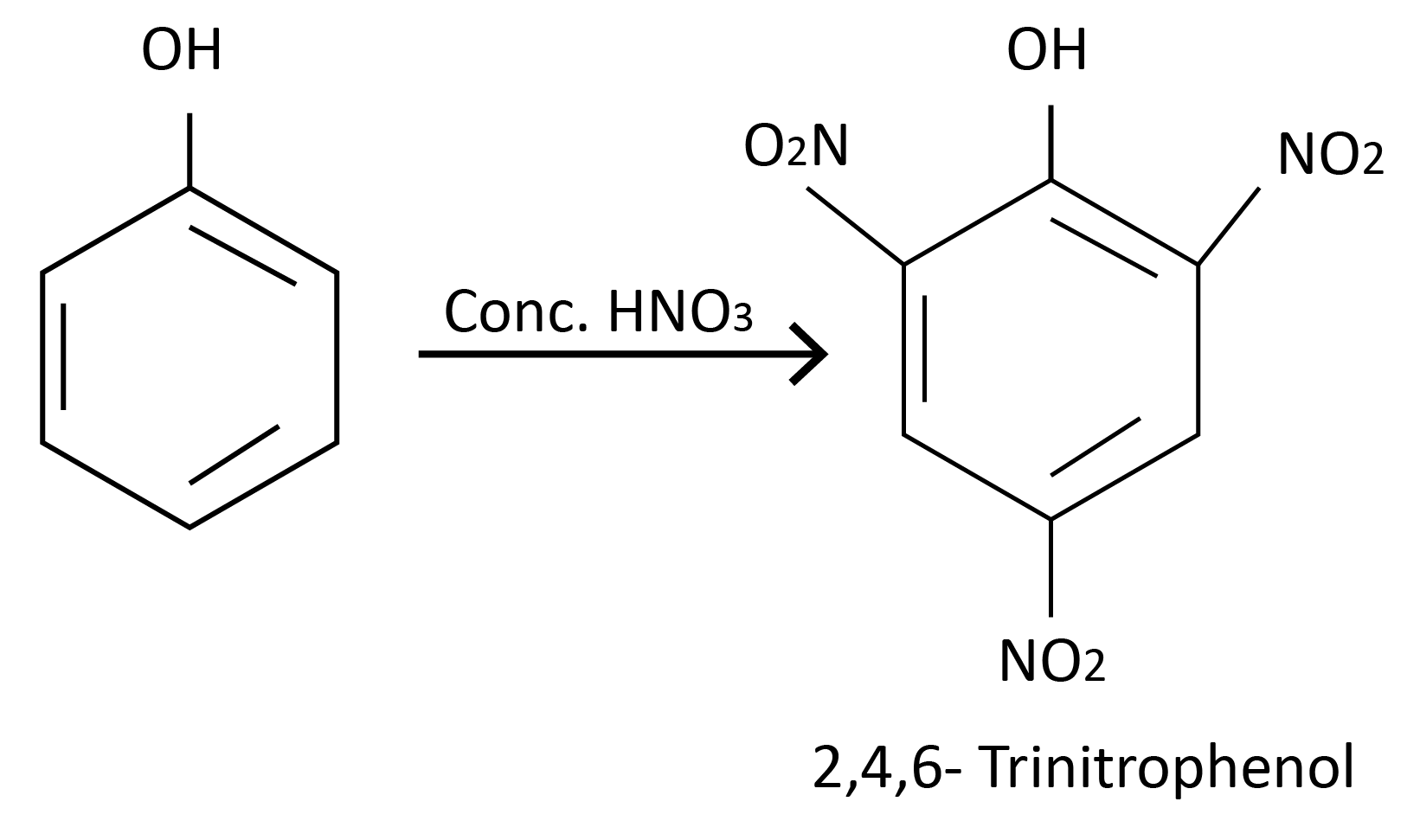
Halogenation:
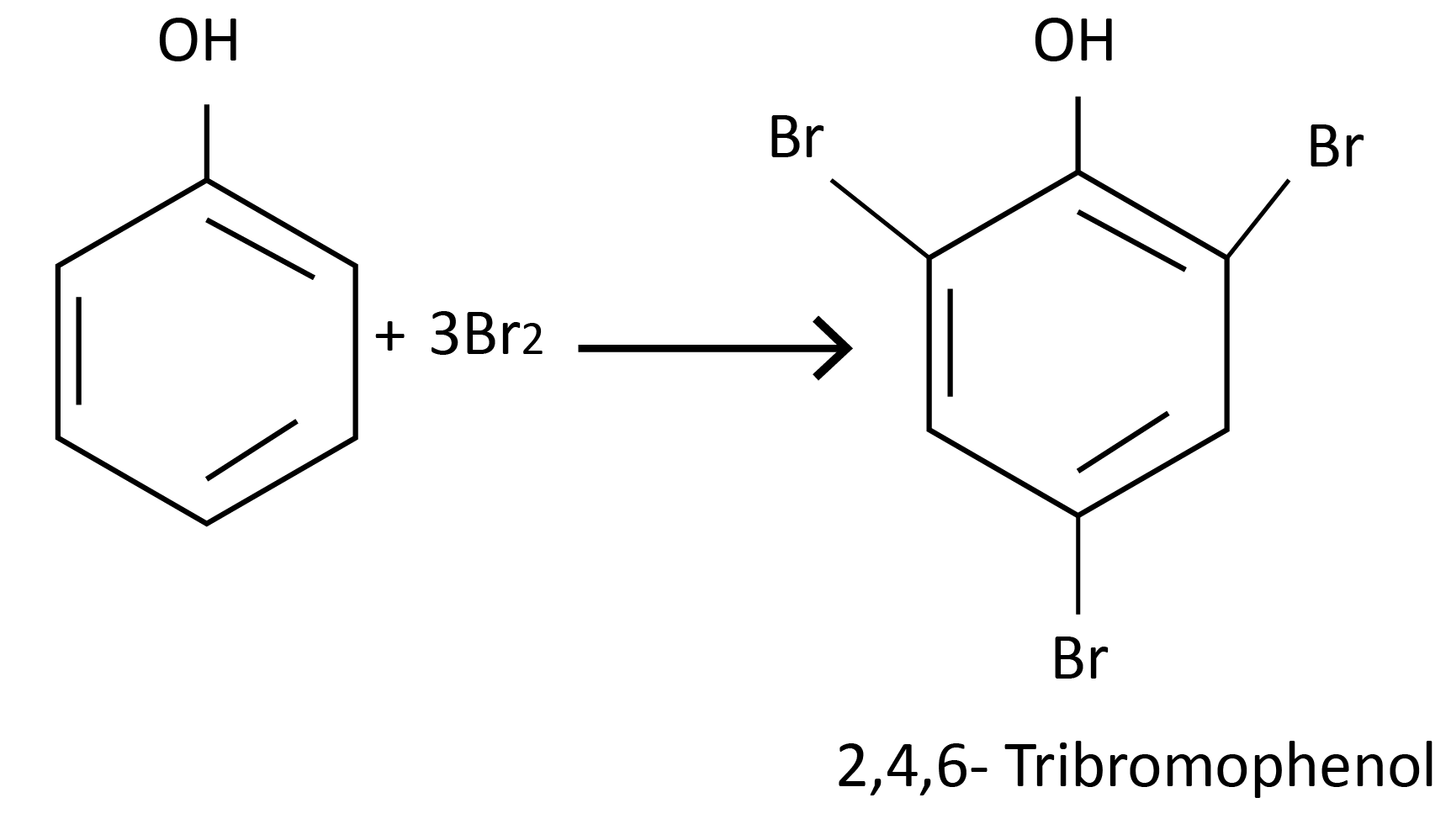
Reactions of Phenol Involving Cleavage of the C-O Bond
Reaction with zinc dust:
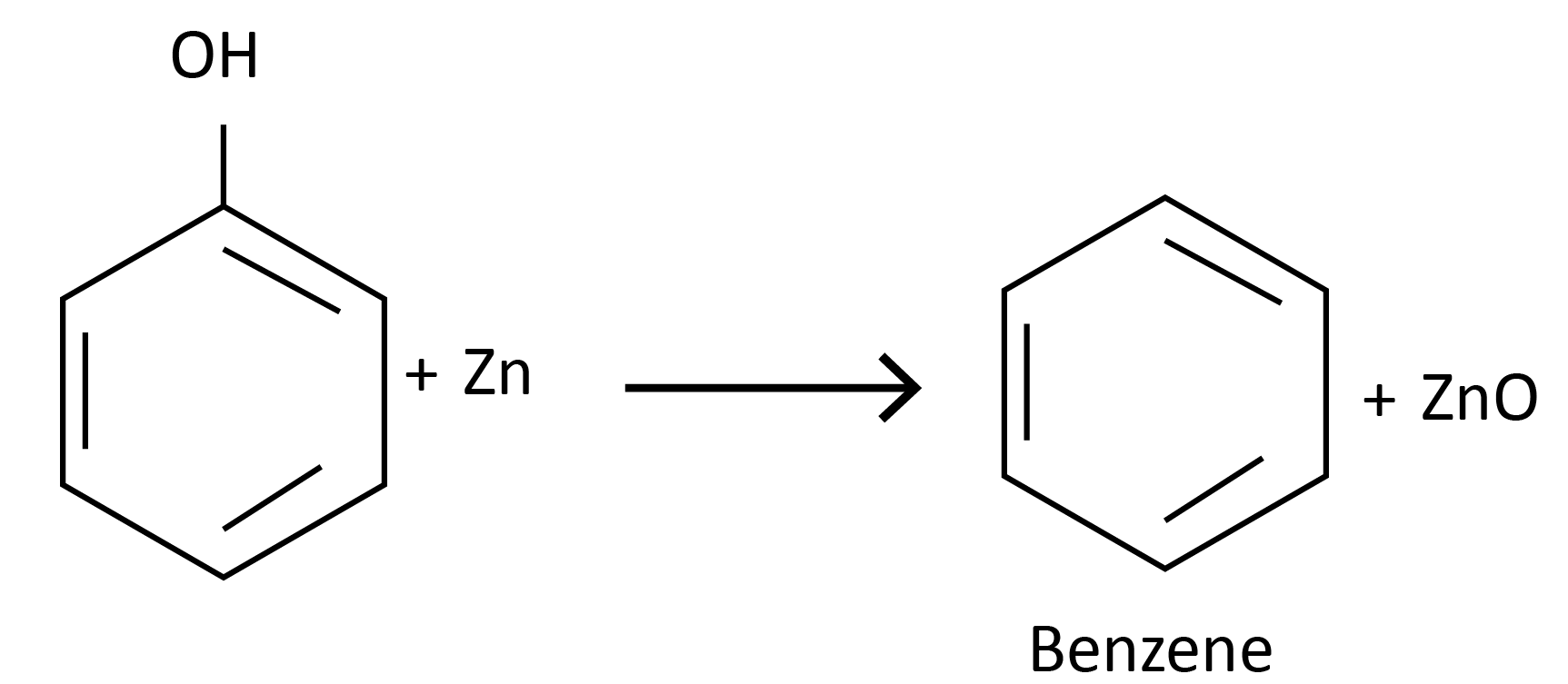
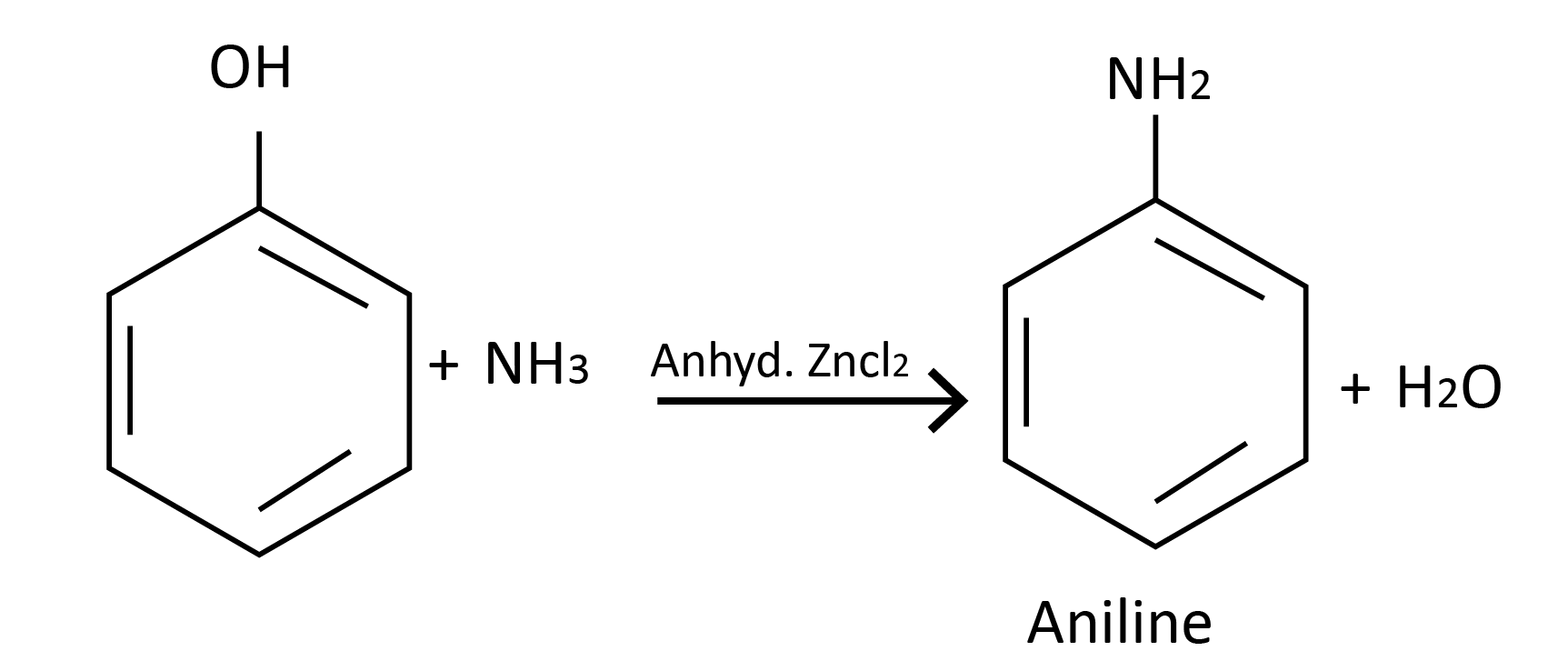
Reaction with ammonia:
Acidity of Phenols
The capacity of phenols to lose hydrogen ions and produce phenoxide ions gives them their acidity.
The benzene ring's sp2 hybridised carbon atom attached directly to the hydroxyl group acts as an electron-withdrawing group in a phenol molecule.
In compared to the hydroxyl group, this sp2 hybridised carbon atom of a benzene ring linked straight to it has a stronger electronegativity.
The electron density on the oxygen atom lowers due to the increased electronegativity of this carbon atom compared to the hydroxyl group connected.
Increased ionisation of phenols is caused by a reduction in electron density, which enhances the polarity of the O-H bond.
The phenoxide ion is produced as a result. The formation of the phenoxide ion is stabilised by the delocalisation of the negative charge caused by the benzene ring's resonance.
Because charge separation occurs during resonance in the case of phenols, the phenoxide ion is more stable than phenols.
Phenols are much more acidic than alcohol but less than carboxylic acids or even carbonic acid.

Effects of Substituents on the Acidity of Phenols
Presence of electron attracting group (e.g., −NO2, −X, −CN,−CHO,−COOH) on the benzene ring increases the acidity of phenol as it enables the ring to draw more electrons from the phenoxy oxygen and thus release easily the proton. Further, the particular effect is more when the substituent is present in the o- or p-position than in the m-position of the phenolic group.
The relative strengths of some phenols (as acids) are as follows :
p-Nitrophenol > o-Nitrophenol > m- Nitrophenol > Phenol
Presence of electron releasing group (e.g., −CH3, −C2H5,−OCH3,−NR2) on the benzene ring decreases the acidity of phenol as it strengthens the negative charge on phenoxy oxygen and thus proton release becomes difficult. Thus, cresols are less acidic than phenol.
However, m-methoxy and m-aminophenols are stronger acids than phenol because of the –I effect and the absence of the +R effect.
m-methoxy phenol > m-amino phenol > phenol > o-methoxy phenol > p-methoxy phenol
Classification of Phenols
Phenols are classified into the following categories depending on the number of hydroxyl groups being present:
(1) Monohydric Phenol: These contain only one hydroxyl group. For example: C6H5OH (phenol).
(2) Dihydric Phenol: These contain two hydroxyl groups. For example: Benzene-1, 2-diol.
(3) Trihydric Phenol: These contain three hydroxyl groups. For example: Benzene-1, 3, 5-triol.
Lederer-Manasse Reaction
One of the most significant phenol reactions is the Lederer-Manasse reaction. In the presence of acid or base, the Lederer-Manasse reaction is described as the condensation of phenol (ArOH) with formaldehyde. The minor product is ortho Benzyl alcohol, whereas the primary product is para Benzyl alcohol. This reaction can take place in both an acidic and alkali media.

Uses of Phenols
In industries, phenol is widely utilised. The following are some of the most common uses for phenol.
In soaps, lotions, and ointments as an antibacterial. "Dettol," a phenol derivative, is a potent antiseptic (2, 4-dichloro-3, 5-dimethyl phenol).
In the production of azo dyes, phenolphthalein, and other similar products.
To make picric acid, which is used as an explosive and to colour silk and wool.
In the synthesis of cyclohexanol, which is used as a solvent for rubber and lacquers and is necessary for the production of nylon.
As an ink preservative.
In the production of bakelite and other phenol-formaldehyde polymers.
In the production of pharmaceuticals such as aspirin, salol, and phenacetin, among others.
To treat caustic wounds produced by crazy dog bites.
Solved Examples From the Chapter
Example 1: Dow’s process is used for the preparation of which of the following?
(A) Esters
(B) Ethers
(C) Phenols
(D) Alcohols
Solution:
In this method chlorobenzene (which is an example of haloarenes which is formed by monosubstitution of the benzene ring) is heated with aqueous NaOH at about 300oC and at high pressure to form sodium phenoxide, which is further acidified by dilute acid like HCl to form phenol.
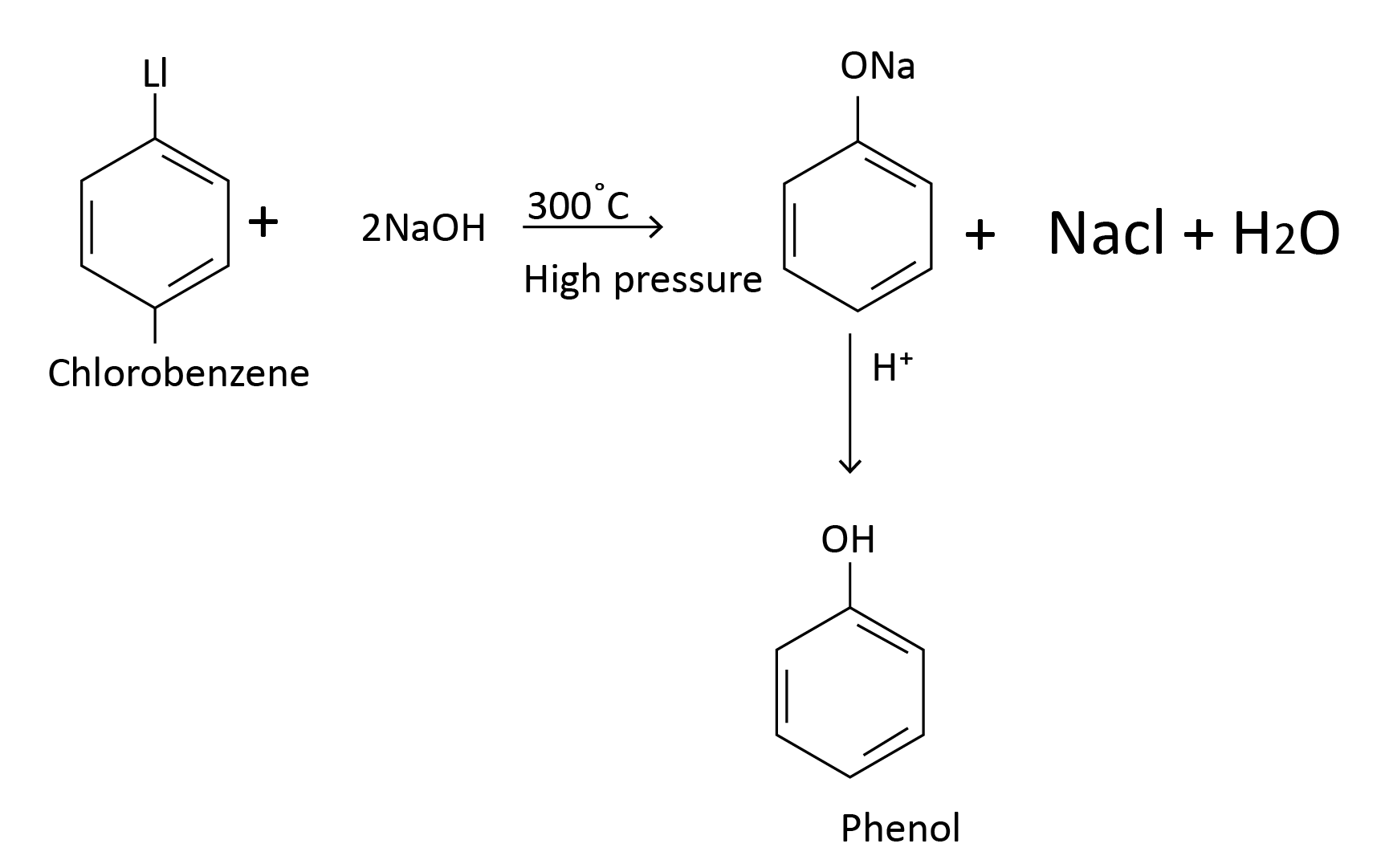
Therefore option (C) is the answer.
Key Point to Remember: The substance we get from Dow's technique is often somewhat acidic in nature. It's an oxidation-reduction process.
Example 2: _________is a reaction of organic chemistry in which an aryl ester is converted to a hydroxy aryl ketone with the assistance of aqueous acid and a Lewis acid catalyst.
(A) Fries Rearrangement
(B) Reimer-Tiemann reaction
(C) Kolbe’s reaction
(D) Dakin Reaction
Solution:
The Fries Rearrangement is an organic chemistry rearrangement process in which an aryl ester is transformed into a hydroxy aryl ketone using aqueous acid and a Lewis acid catalyst. An acyl group from the phenolic ester is transferred to the aryl ring in the Fries Rearrangement process. It's also worth noting that the Fries rearrangement is selective for the ortho and para locations of the aryl ring, implying that the acyl group is connected to the ortho or para position of the aryl ring. Hence option (A) is correct.
Key Point to Remember: The acyl group's carbonyl oxygen forms a compound with a Lewis acid catalyst (Aluminium chloride). Because carbonyl oxygen contains more electrons than other oxygens, it is a superior Lewis base. As a result, the formation of this combination with carbonyl oxygen is preferred over complex formation with phenolic oxygen.
Solved Questions from the Previous Year’s Question Papers
Question 1: Arrange the following compounds in order of decreasing acidity :
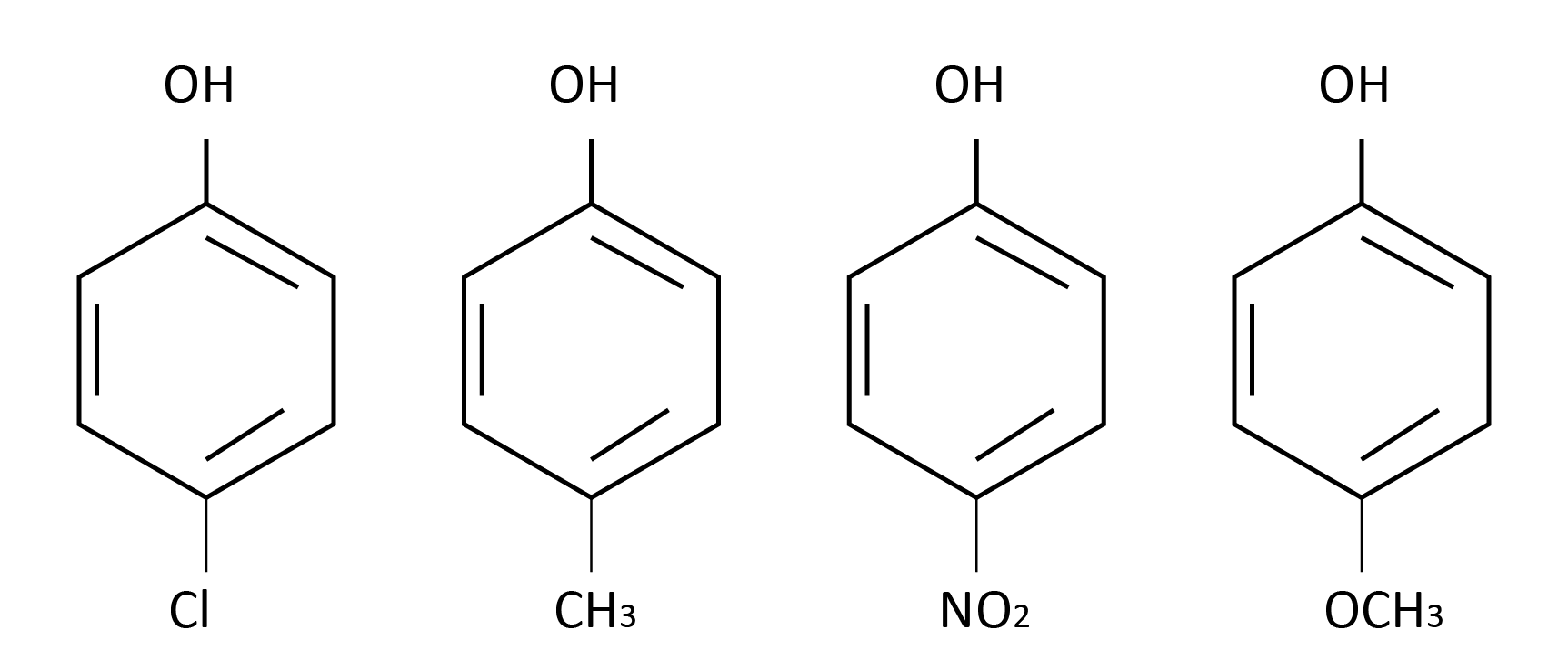
A) I > II > III > IV
B) III > I > II > IV
C) IV > III > I >II
D) II > IV > I > III
Solution:
The capacity to contribute H+ ions can be described as the acidity of a substance. Stronger acids are those that can easily transfer hydrogen ions, whereas weaker acids cannot. It's worth noting that the electron-releasing groups are the ones who supply the electrons. The electron density of oxygen rises as a result. The O-H bond will not attract electrons since oxygen has a high electron density. As a result, the O-H connection will be strong and unlikely to break.
The electron withdrawing groups, on the other hand, will attract electrons to themselves. As a result, the electron density on the oxygen atom will fall, and the oxygen will draw electrons from the O-H bond, weakening it. As a result, it will be easy to give electrons. Both effects are seen in the Cl group. However, in comparison to ordinary phenol, it will remove electrons and hence have an electron-withdrawing action, increasing acidity.
The methyl group (+I) on the second molecule acts as an electron donor. As a result, the acidity will be reduced.
The nitro group, which is a powerful electron-withdrawing group, is present in the third molecule. As a result, the maximum acidity will rise.
The methoxy group on the fourth molecule has a substantial electron releasing impact. As a result, acidity will be reduced.
As a result of our observations, we can state that the acidity of the following compounds decreases in the following order: III > I > II > IV.
Therefore option (B) is the answer.
Trick: The electron releasing groups will give electrons and as a result, the electron density on oxygen will increase, and thus, the H+ ion will be difficult to donate. But the electron-withdrawing groups will attract the electrons toward themselves and thus reduce electron density on the oxygen atom. As a result, the electron density on oxygen atoms will decrease and hence the H+ ion will be easily donated.
Question 2: Which will undergo a Friedel-Craft's alkylation reaction?
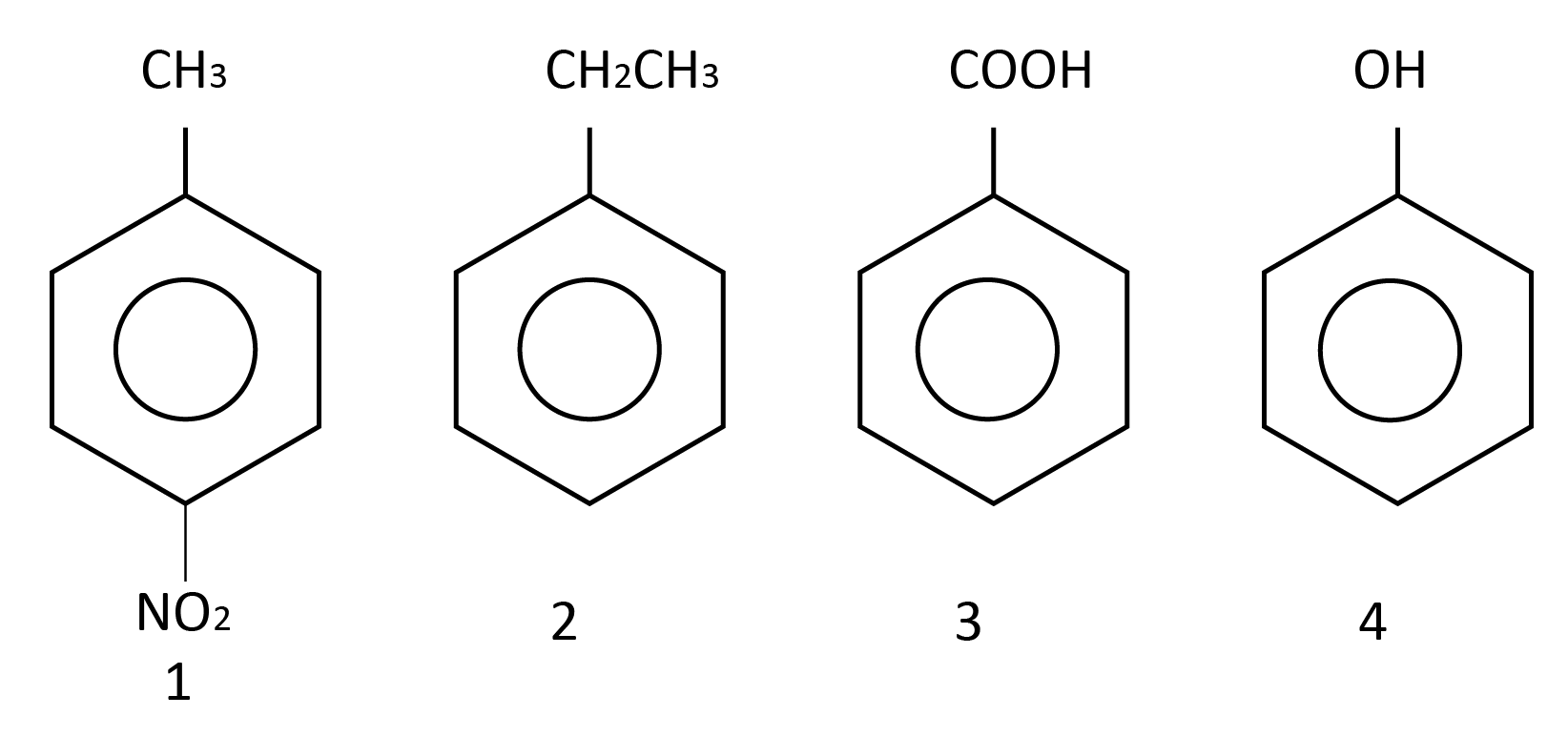
A) 1, 2 and 4
B) 1 and 3
C) 2 and 4
D) 1 and 2
Solution:
The Friedel–Crafts reactions were invented by Charles Friedel and James Crafts to attach substituents to aromatic rings. Friedel–Crafts alkylation reactions in the form of Friedel– Crafts reactions. Electrophilic aromatic substitution is used in this case. In this process, the rings which are electron-rich give this type of reaction. Hence option (C) is correct. From here alkylation on ortho or para position occurs because of more electron density.
Trick: Friedel-Crafts Alkylation is defined as the substitution of an alkyl group for an aromatic proton. With the assistance of a carbocation, an electrophilic assault on the aromatic ring is carried out.
Question 3:
$\text{Phenol}\xrightarrow{\text{NaN}{{\text{O}}_{\text{2}}}\text{/}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}}\text{B}\xrightarrow{{{\text{H}}_{\text{2}}}\text{O}}\text{C}\xrightarrow{\text{NaOH}}\text{D}$
Name of the above reaction is
A) Liebermann’s reaction
B) Phthalein fusion test
C) Reimer-Tiemann reaction
D) Schotten-Baumann reaction
Solution:
When phenol is combined with NaNO2 and concentrated H2SO4, it produces a dark green or blue colour that becomes red when diluted with water. In the presence of NaOH / KOH, the generated substance returns to its original green or blue colour.
So, the above reaction is Liebermann’s reaction. Hence option (A) is correct.

Trick: Remember the concept behind Liebermann’s reaction.
Practice Questions
Question 1: Oxidation of which of the following by air in presence of vanadium pentoxide gives phenol
A) Toluene
B) Benzene
C) Benzaldehyde
D) Phenylacetic acid
Answer: (B) Benzene
Question 2: Which of the following does not form phenol or phenoxide
A) C6H5Cl
B) C6H5COOH
C) C6H5N2Cl
D) C6H5SO3Na
Answer: (B) C6H5COOH
Conclusion
The structural, physical, and chemical aspects of phenol are all discussed here. Students may learn all about the basics and advances of phenols. Hence, this is important not only for competitive exams like JEE or NEET but also for a better understanding of the concepts being involved.
FAQs on JEE Chapter - Phenols
1. Why is ortho hydroxybenzoic acid more acidic compared to para hydroxybenzoic?
In salicylic acid, the hydroxyl group is closer to the carboxylic acid group than in para-hydroxy benzoic acid. Acidity is raised in two ways as a result of this.
The ionized component of the carboxylic acid group is stabilised by the electronegative hydroxyl group. Every carboxylic acid undergoes this process, in which nearby hydrogen atoms are replaced with more electronegative atoms such as fluorine, chlorine, or oxygen.
The hydrogen ion is gently drawn away from the carboxylic acid group by the negative partial charge of the oxygen atom, allowing dissociation.
Based on these findings, we may conclude that O-Hydroxybenzoic acid is more acidic than p-hydroxybenzoic acid due to its more stable conjugate base.
2. What is the structure of phenol?
Because phenol has a ring structure, the carbon atom in the ring (with alternating double bonds) is sp2 hybridised. The –OH group is connected to the aromatic ring's sp2 hybridized carbon atom. When the sp3 orbital of oxygen and the sp2 hybridized orbital of carbon in the aromatic ring meet, the C–O bond is created.
3. Why phenol is acidic?
Phenols are acidic in nature, this is because they release the H+ ions from the hydroxyl group. When the OH group is involved in resonance, a proton is released, and the oxygen receives a partial positive charge. This allows the H+ ions to readily migrate out, making phenols a Bronsted acid. Because the phenoxide ion is stabilised by resonance, phenols are more powerful acids than their alcohol counterparts. This facilitates the elimination of H+ ions from phenols.























