




What is an Elimination Reaction?
A molecule of hydrogen halide is eliminated and an alkene is formed when a haloalkane is heated with a concentrated alcoholic solution of potassium hydroxide. This reaction is an example of an elimination reaction. The hydrogen in the alkyl halide that is eliminated comes from a beta-carbon (the carbon atom next to the one that carries the halogen) and the halogen comes from the alpha-carbon (i.e., the carbon atom which carries the halogen). Since the hydrogen atom is lost from the beta-carbon during this reaction, it is referred to as a beta-elimination reaction.
It is the principal process by which organic compounds containing only single carbon-carbon bonds (saturated compounds) are transformed into compounds containing double or triple carbon-carbon bonds (unsaturated compounds). Elimination reactions are the opposite of addition reactions. β–carbon must be bonded to at least one hydrogen for elimination to occur.
E2 Elimination Reaction
There are two types of elimination. The E2 mechanism is bimolecular elimination. On the other hand, the E1 mechanism consists of unimolecular elimination. The timing of bond cleavage and formation differs between the E2 and E1 mechanisms, which is analogous to the SN2 and SN1 mechanisms. E2 and SN2 reactions share some characteristics, as do E1 and SN1 reactions. Elimination reactions are typically distinguished by the types of atoms or groups of atoms that leave the molecule. As a result, there are two primary methods involved in this type of reaction: dehydration and dehydrohalogenation.
Dehydration involves the removal of a water molecule from compounds such as alcohol. This method is also known as the Beta elimination reaction because the leaving group and H are placed on neighbouring carbon atoms. On the other hand, dehydrohalogenation involves the removal of a hydrogen atom as well as a halogen atom.
E2 Reaction Mechanism
Let us consider the reaction of 2-Bromo 2-methyl butane with a strong base to yield 2-methyl-2-butene as the main product of the elimination reaction. The E2 mechanism is a one-step elimination process with a single transition state. These reactions are observed in primary or secondary substituted alkyl halides. Both the alkyl halide and the base influence the rate of reaction. As a result, it is classified as a bimolecular elimination reaction. This reaction follows second-order kinetics. Here is an example of an E2 elimination reaction.
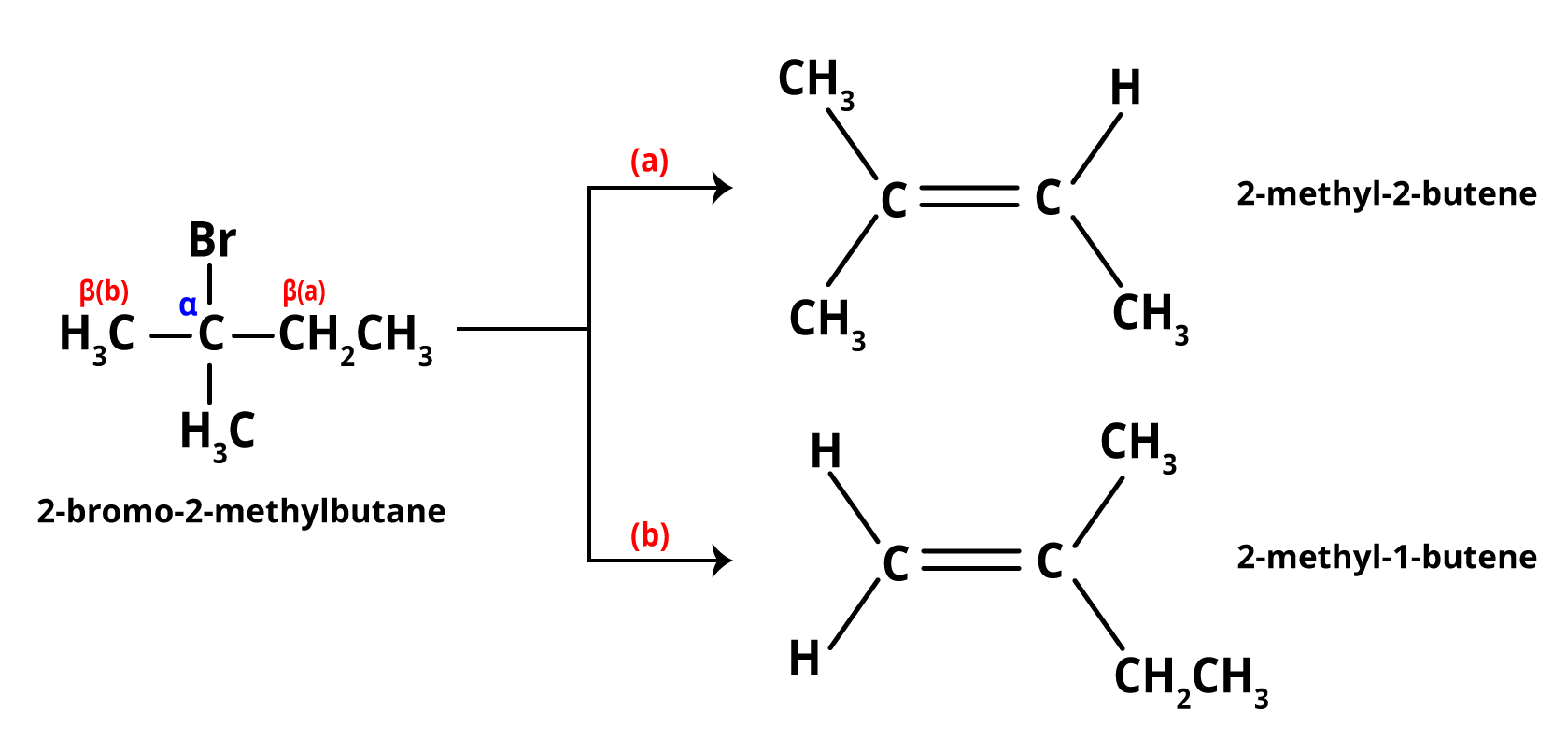
Image: E2 Reaction Examples
The E2 reaction transition state indicates that the C-H and C-Br bonds are partially broken while the O-H and C=C bonds are partially formed. However, in the E2 mechanisms, a base is a rate-determining step that has a significant impact on the mechanism.
Rate = k$[RX][Base]$ is the rate of the E2 reaction.
As a result, the reaction rate is affected by both the substrate and the base involved. A stable alkene is the major product.
The Stereochemistry of the E2 Reaction
The transition state of an E2 reaction is made up of four atoms from the substrate connected in a plane (one hydrogen atom, two carbon atoms, and the leaving group, X). In two ways, the C—H and C—X bonds can be coplanar. In an antiperiplanar configuration, E2 elimination is perhaps the most common. This configuration allows the molecule to react in the lower energy staggered conformation, permitting the incoming base and leaving the group to be separated. The anti-orientation also allows for rapid interaction between the bonding electrons of the C-H bond and the antibonding orbital of the C-X bond.
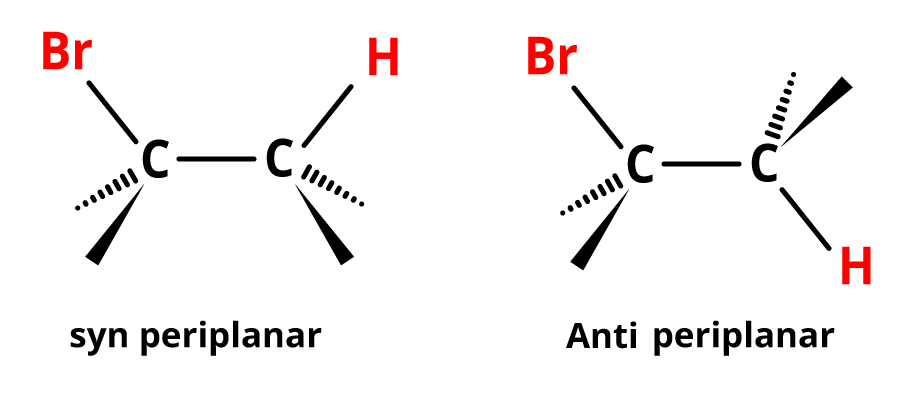
Image: Stereochemistry of Elimination Reaction
Saytzeff Rule
Saytzeff's Rule is also known as Zaitsev's Rule, Saytzeff's Rule, and Z-rule. Alexander Zaitsev, a Russian chemist, studied various elimination reactions and discovered a general pattern in the resulting alkenes. During the elimination reaction, a proton is removed from a carbon atom with fewer substituents. If the halogen is present on any carbon atom in the chain, the alkyl halide can dehydrohalogenate in two or more directions, depending on the number of different types of beta hydrogens available. In all of these cases, the more highly substituted alkene (one with fewer hydrogen atoms on the doubly bonded carbon atoms) is the main product of dehydrohalogenation.
Conclusion
In Organic Chemistry, alkenes are the main products of elimination reactions, which are of two types, E1 and E2. Their mechanism is similar to substitution reactions SN1 and SN2. Stereochemistry of trans alkenes is more stable and hence a major product. In Organic Chemistry, E1 reactions are a type of two-step elimination reaction, whereas E2 reactions are a type of one-step elimination reaction. Unimolecular eliminations are the reaction mechanisms of E1 reactions. Bimolecular eliminations are the reaction mechanisms of E2 reactions.
FAQs on E2 Reaction with Mechanism and Examples for JEE
1. What do you understand about Hoffman's elimination reaction?
It is the process of treating quaternary ammonium with excess methyl iodide, then treating the resultant chemical with silver oxide, water, and heat, to produce tertiary amines. This process is also called Hoffman's exhaustive methylation. After the hydroxyl anion replaces the iodine, the alkene is created through an elimination reaction. According to the Hofmann rule, when it comes to asymmetrical amines, the primary alkene product is the least substituted and least stable product. The reaction is as follows:
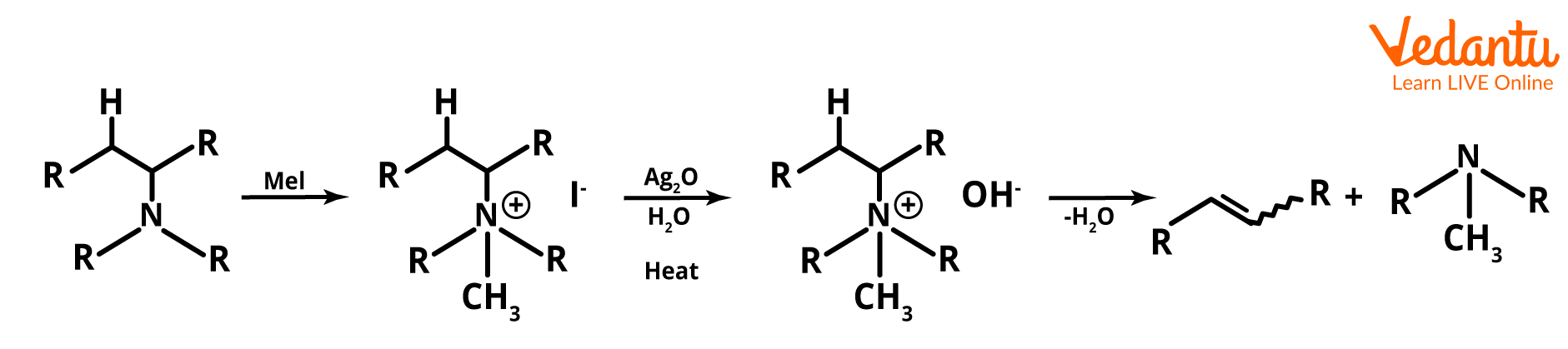
Image: Hoffman's elimination reaction example
2. What are the similarities and differences between E1 and E2 reactions?
E1 and E2 reactions are both elimination reactions. Polar protic solvents promote E1 reaction by stabilising carbocation. Secondary alkyl halides exhibit two very different types of reactions. If the alkyl halide includes better-leaving groups, the rate of both reactions increases.
The E1 reaction occurs in the absence of bases or the presence of weak bases. In the presence of strong bases, E2 reactions occur. The mechanism E1 involves the formation of carbocations. Transition states are intermediate stages of E2 reaction. Tertiary alkyl halides and some secondary alkyl halides are prone to E1 reactions. Primary alkyl halides and some secondary alkyl halides are prone to E2 reactions.

































