




Mastering Melting and Boiling Point Experiments: Key Concepts & Tips for Class 9 Chemistry Exams
While boiling the water, the water begins to warm up as the temperature rises slowly because the kinetic energy is rising at the same time. The intermolecular distance surrounding the water molecules continues expanding due to this increase in kinetic energy, and the molecules can now move about more freely. When the temperature reaches a specific point, the intermolecular space expands to the point where the water molecules separate from one another and vaporize. This explains the boiling point temperatures of the water. Ice (solid), water (a liquid), and gas (vapour) are all different states of the element water.
Table of Content
Introduction
Aim
Procedure
Result
Aim
To determine the water's boiling point and the melting point of ice.
Apparatus Required
Ice
Beaker
Wire gauze
Burner
Thermometer
Clamp stand
Tripod Stand
Boiling tube
Theory
The melting point decreases when the pressure increases, and the boiling point increases when the pressure increases. The ice melting point is 0 °C, meaning that at that temperature, the forces of contact between the molecules of solid H20 can be melted, turning the ice into water. Since there are fewer forces of interaction in the liquid form, water is a liquid at ambient temperature. Water has a boiling point of 100 °C, which means that at sea level, the water's vapour pressure equals the air pressure at this temperature.
The heat energy known as latent heat of fusion is acquired during ice melting and is preserved in the water produced. Latent heat of fusion is defined as the amount of heat energy needed to transform 1 kilogram of a solid into a liquid at its melting point.
Procedure
To Determine the Boiling Point of Water Experiment
Add a couple of pumice stones to a boiling tube of 25–30 ml of water.
As illustrated in the boiling tube diagram below, attach and set the thermometer atop the water in the boiling tube and note the temperature.
Underneath the boiling tube, place a Bunsen burner. Measure and record the temperature until the water boils.
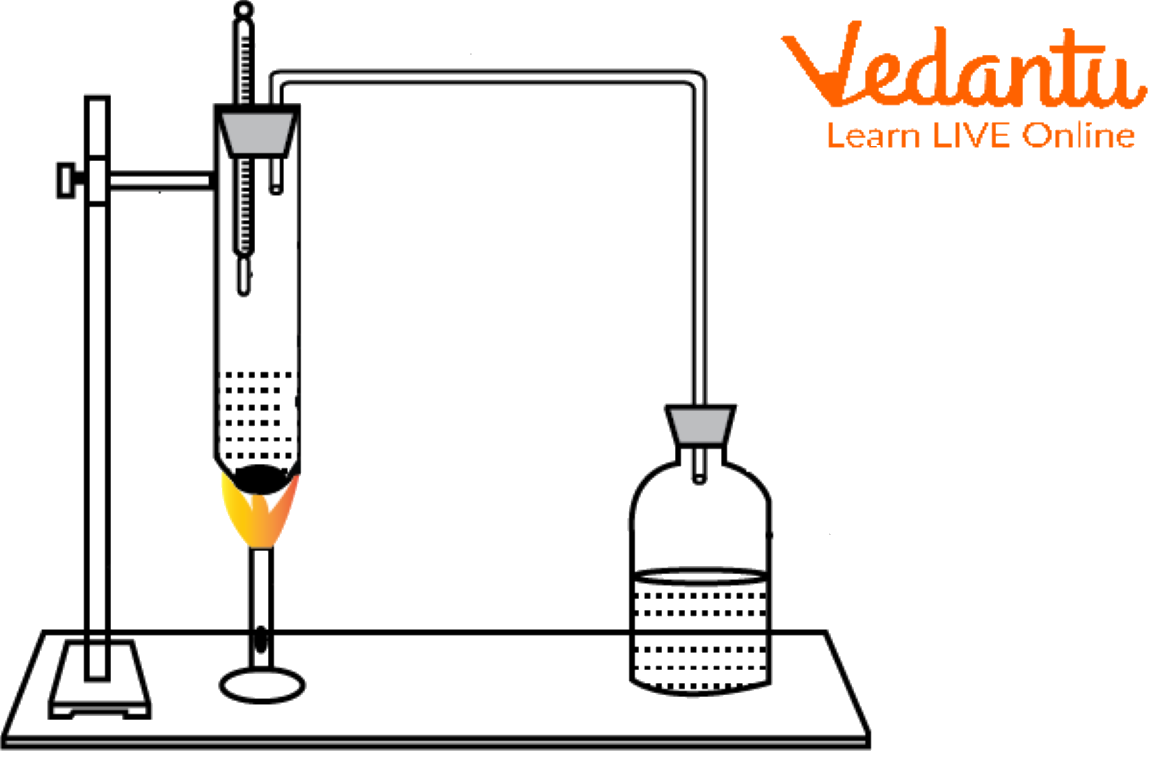
Boiling Tube
To Determine the Melting Point of Ice Experiment
In a beaker, load it's half using distilled water-derived dried crushed ice.
Drop a thermometer off the clamp stand so that the ice entirely encircles the thermometer bulb.
Observe and record the thermometer readings per 1 min until the ice dissolves.
The melting point apparatus diagram is provided below:

Melting Point Apparatus
Observations
Result
The melting point of ice is 0℃
The boiling point of water is 100℃
The temperature stays steady once the ice melting process starts and the boiling point is attained.
Precautions
While heating or boiling, utilize a proper clamp stand. Boil the water by rotating the flame.
The thermometer's bulb needs to be just barely above the water or ice.
Pick a thermometer of higher calibre with an easily interpretable calibrated scale. The sides of the boiling tube or beaker must not be touched by the thermometer.
Lab Manual Questions
1. Why is mercury an excellent thermometer material?
Ans: Mercury in a liquid condition at normal temperature possesses the maximum expansion coefficient. Thus, even a small shift in temperature is noticeable.
2. Why are pumice stones added while boiling water?
Ans: While boiling water, energy is released in the form of bubbles. If no bubbles appear, the water could be too hot and explode.
3. To determine the boiling point, why do we place a two-holed cork in the flask with a rounded bottom?
Ans: To enable the steam to exit the flask because if it doesn't, the rounded bottom flask could explode due to excessive internal pressure.
4. Why does the thermometer bulb placed on top of the water's surface for estimating the water's boiling point?
Ans: To determine accurate water’s boiling point since steam simply comprises water vapour, and any non-volatile impurities that may exist in the water will have no effect.
Viva Questions
1. Define melting point?
Ans: The point at which the substance shifts from solid to liquid.
2. Define boiling point?
Ans: The temperature at which a liquid’s vapour pressure equals the gas pressure.
3. Who invented the thermometer?
Ans: Daniel Gabriel Fahrenheit
4. Thermometer is said to be accurate if Fahrenheit shows
Ans: 32oF
5. Why do individuals spray NaCl on Ice in colder regions?
Ans: The melting of ice is easier.
6. What is the relationship between boiling point and vapour pressure?
Ans: Inversely proportional.
7. Why does ice float on water?
Ans: Water has a higher density compared to ice.
8. The density of water is maximum at
Ans: 4oC
9. In laboratories to determine the boiling point of water, which type of water is used
Ans: distilled water
10. Steam or water, which possesses higher heat energy?
Ans: steam
Practical Based Questions
1. What is the boiling point of impure or hard water?
100oC
Above 100oC
Below 100oC
Neither of the three option
Ans: The boiling point of impure or hard water is above 100oC.
2. What is the boiling point of pure water in Kelvin?
373.15K
273.15K
243K
173.15K
Ans: The boiling point of pure water in kelvin 373.15K.
3. What is the melting point of pure water in Kelvin?
373K
273K
243K
173K
Ans: The melting point of pure water in kelvin is 273 K.
4. When a thermometer is taken from the melting ice to the water which is warm, (2/5) th of the space connecting the upper and bottom point sets is reached by the mercury level. What is the water's temperature?
217K
220K
313.15K
330K
Ans: The temperature of water is 313.15K
5. Why does the temperature of the ice not increase when heated constantly?
Heat goes out of the system
Heat is not used
Formation of latent heat
Heat remains inside the system
Ans: The temperature of ice does not increase when heated constantly due to the formation of latent heat.
6. There are 20 splits between 10 °C and 20 °C on a thermometer. What is the thermometer's lowest count?
0.5
0.1
1
2
Ans: The thermometer's lowest count is 0.5.
7. When NaCl is added to the water what happens to the boiling point of water?
Increases
Decreases
Remains constant
None of the three option
Ans: When NaCl is added to the water the boiling point of the water increases.
8. What is the boiling point of water in hill stations?
100o C
Less than 100o C
Greater than 100oC
None of the three option
Ans: The boiling point of water in hill stations is less than 100oC.
9. In the chemistry labs, which of the following will be chosen to determine the melting point of ice?
Slab of ice
Dry crushed cubes of ice
Ice cubes
Ice cubes added to the water
Ans: In chemistry labs dry crushed cubes of ice will be chosen to determine the melting point of ice.
10. At what temperature does Ice and water co-exist in atmospheric pressure?
0o C
Below 0o C
Above 0o C
None of the three option
Ans: The ice and water co-exist in atmospheric temperature at 0o C.
Conclusion
This chemistry experiment article helps to determine the melting point of ice and boiling point of water with a simple and easy procedure. The solid and liquid phases are in equilibrium at the melting point. A particle's melting point is often reported at a standard pressure because it relies on pressure. The temperature where the water turns into a vapour and the pressure of the surrounding air equals that of the water is known as the boiling point of water. This experiment verifies the melting point of ice is 0℃ and the boiling point of water is 100℃
FAQs on Hands-on Guide to Melting Point of Ice and Boiling Point of Water for CBSE Class 9 Chemistry (2025-26)
1. What are the standard melting point of ice and the boiling point of water? State the values in both Celsius and Kelvin scales for the CBSE Class 9 exam.
The standard melting point of ice is 0° Celsius (273.15 Kelvin). The standard boiling point of water is 100° Celsius (373.15 Kelvin). These values are important to remember as they are measured at standard atmospheric pressure (1 atm).
2. For a Class 9 practical exam, what are some important precautions to take while determining the boiling point of water?
For the CBSE Class 9 practical exam for the 2025-26 session, important precautions to ensure an accurate result include:
- The thermometer bulb should not touch the bottom or sides of the beaker to measure the temperature of the water, not the glass.
- Continuously stir the water to ensure uniform heating throughout the liquid.
- Keep your eyes level with the mercury to avoid parallax error when reading the thermometer.
- Add a few pieces of pumice stone to the water before heating to prevent 'bumping' and ensure smooth boiling.
3. Define latent heat of fusion and latent heat of vaporisation. Which one has a higher value for water?
This is a frequently asked question in Class 9 Chemistry exams.
- Latent Heat of Fusion: This is the amount of heat energy required to change 1 kg of a solid into a liquid at its atmospheric pressure and melting point, without any change in temperature. For ice, this value is approximately 3.34 x 105 J/kg.
- Latent Heat of Vaporisation: This is the amount of heat energy required to change 1 kg of a liquid into a gas at its atmospheric pressure and boiling point, without any change in temperature. For water, this value is approximately 22.6 x 105 J/kg.
The latent heat of vaporisation for water is significantly higher than its latent heat of fusion.
4. Why does the temperature remain constant during the melting of ice or boiling of water, even when heat is continuously supplied?
The temperature remains constant because the supplied heat energy, known as latent heat, is entirely used to overcome the forces of attraction between the particles of the substance. During melting, this energy (latent heat of fusion) breaks the bonds holding the ice crystals together. During boiling, the energy (latent heat of vaporisation) overcomes the intermolecular forces in liquid water. Only after the entire substance has changed its state does the temperature begin to rise again.
5. How does an increase in pressure affect the melting point of ice and the boiling point of water? This is a frequently asked HOTS question.
Understanding the effect of pressure is a key higher-order thinking skill (HOTS) concept:
- Effect on Melting Point of Ice: An increase in pressure lowers the melting point of ice. This is an exception because ice is less dense than water, and applying pressure favours the formation of the denser liquid state at a lower temperature.
- Effect on Boiling Point of Water: An increase in pressure raises the boiling point of water. The external pressure on the liquid's surface makes it more difficult for molecules to escape into the gaseous phase, thus requiring more heat energy (a higher temperature) to boil.
6. Why are burns from steam at 100°C considered more severe than burns from boiling water at the same temperature?
Burns from steam at 100°C are more severe due to the latent heat of vaporisation. When steam touches the skin, it first condenses into water at 100°C, releasing a large amount of extra heat energy onto the skin. The skin is then burned by both this released latent heat and the heat from the resulting hot water. Boiling water, in contrast, only transfers its own heat without this additional energy from the phase change.
7. How do impurities, such as salt, affect the melting point of ice and the boiling point of water?
Adding impurities has a predictable effect, which is an important concept for Class 9 exams:
- Effect on Melting Point: Impurities like salt lower the melting point of ice. This is called the depression of freezing point and is the reason salt is used on icy roads to make them safer.
- Effect on Boiling Point: Impurities raise the boiling point of water. This is called the elevation of boiling point, as the dissolved particles interfere with the escape of water molecules, requiring more energy to boil.
8. Explain why it is important to stir the water continuously while heating it to determine its boiling point in a lab experiment.
It is crucial to stir the water continuously for two main reasons that are important for accurate practical exam results:
- Uniform Temperature Distribution: Stirring ensures that the heat is distributed evenly throughout the water. Without it, the bottom layer would get hotter than the top, leading to an inaccurate boiling point measurement.
- Preventing Bumping: Stirring, along with adding pumice stones, helps prevent 'bumping'—a sudden, violent boiling that can splash hot water and cause an unstable thermometer reading, making the experiment unsafe and results unreliable.































