




An Overview of Class 9 Chemistry Law Of Conservation Of Mass Experiment
Antonio Levosier in 1789 is the scientist who gave the Law of conservation of mass. He states that we can not create a mass or destroy it, but it can change its form from one form to another form and the total mass of a compound remains constant in a chemical reaction.
In our daily life, we can see the conservation of mass while preparing lemonade. In a lemonade, we mix the sugar in water. Here the mass of both ingredients remains the same in the lemonade, only its form changes.
The Law of conservation of mass states that matter can be created or destroyed in a chemical reaction. In a campfire, the wooden sticks burn down into ash. We assume that the mass has reduced in the campfire, but the mass of the whole system remains the same. The wood's mass is equal to the mass of the ash and the smoke or gas produced.
Table of Content
Aim
Conservation of Mass during Physical Change
Conservation of Mass in Chemical Change
Conservation of Mass in Rearrangement
Result
Aim
To verify the law of conservation of mass in a chemical reaction through the experiment of two different sets of chemicals:
Set 1-Barium chloride and Sodium Sulphate
Set 2- Silver Nitrate and Sodium Chloride
Apparatus Required
Two watch glasses
Beaker
Weighing Balance
Glass Rod
Barium chloride and sodium sulphate
Conical Flask
Ignition Tube
Cork
Theory:
The Law of conservation of mass says matter can not be created or destroyed in a chemical reaction, it changes its forms from one form to another form.
Conservation of Mass during Physical Change: When mass changes from solid to liquid or gas, its mass remains the same. For example-When ice melts it converts into water and the water of heating changes into the water vapours, but the mass of matter in all three states remains the same.
ICE (Solid) ⇌ Water (Liquid) ⇌ Water Vapour (Gas)
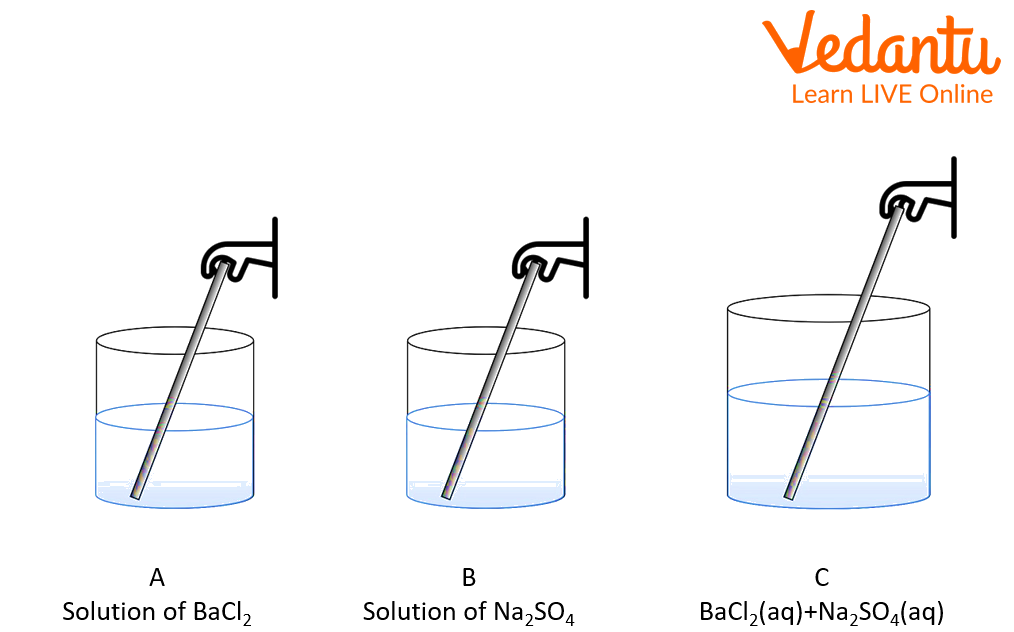
Conservation of Mass in Chemical Change: Chemical Change: In a chemical reaction, the overall mass of a reactant is equal to the mass of the product form. Example: When barium chloride is reacted with sodium sulphate, it produces a white precipitate of barium sulphate and sodium chloride. In this reaction, the mass of the reactant and product remains the same.
BaCl2(aq)+Na2SO4(aq)→BaSO4(s)+2NaCl(aq)
Conservation of Mass in Rearrangement: On heating calcium carbonate, it produces carbon dioxide and calcium oxide. The overall mass of calcium carbonate and products remains the same in this reaction.
CaCO3 → CaO + CO2
Conservation of Mass in Nuclear Reaction: In a nuclear reaction, the atoms change by changing the number of protons. For example-Uranium undergoes nuclear fission to produce Barium and krypton.
Procedure:
The Experiment of Conservation of mass Barium Chloride and Sodium Sulphate
Take the weight of two watch glasses by physical balance.
Pour 50 ml of distilled water into the beaker of 100 ml.
Weigh 3.6 gm of barium chloride (BaCl2.2H2O) in a watch glass and then mix it in 50 ml of distilled water and name it A.
Weigh 8.05 gm of sodium sulphate in another watch glass and mix in a 50 ml distilled water beaker and label it B.
Now take another beaker of 150ml and take the weight of that beaker using balance and label it C.
After that, mix the solutions A and B in beaker C using a glass rod.
A white precipitate starts forming in beaker C.
Now weigh the beaker C after the reaction. Compare the mass of all three beakers A, B, and C.
Reaction:
BaCl2 (aq)+ Na2SO4 (aq) → BaSO4 (S) + 2NaCl (aq)
Law of Conservation of Mass
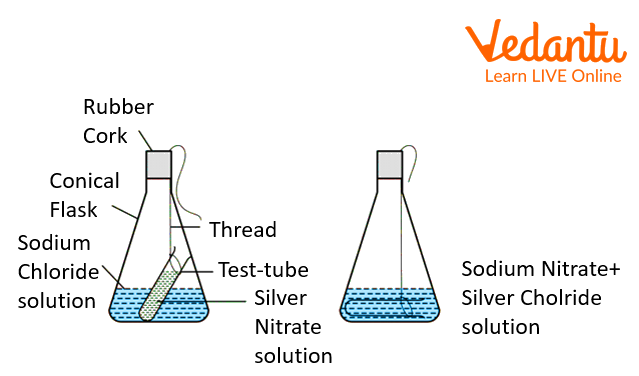
The Experiment of Silver Nitrate and Sodium Chloride:
Make a 5% solution of silver nitrate and sodium chloride separately.
Fill the sodium chloride solution in the conical flask.
Then fill lead nitrate in an ignition tube.
Now suspend the ignition tube in the conical flask in such a way that the ignition tube does not flow into the flask.
Seal the flask with a cork.
Take the Weight of the flask now and note the reading.
Then tilt the conical flask so that silver nitrate mix with sodium chloride.
Now reweight the conical flask and note the reading.
Reaction:
AgNO3(aq) + NaCl (aq) → NaNO3 (aq) + AgCl (s)
Law of Conservation of Mass
Observation:
Observation 1:
Observation 2-
We observe that the white precipitate of silver chloride is formed. The weight of the conical flask before the reaction is the same as the weight of the conical flask after the reaction.
Result:
The weight of reactant barium chloride and sodium sulphate before the reaction is 111.65 gm and after the reaction, the weight of the product is also 111.65 gm.
The white precipitate of the silver chloride is formed. The weight of the reactant in the second experiment of silver nitrate and sodium chloride is the same as after the reaction.
Precaution:
Ensure that the flask is measured separately before the reaction.
Clean the conical flask or beaker properly.
The weight of the mass of the reactant and product was measured carefully.
It is ensured that the weight in the weighing balance is zero before taking the reading.
Stir the solution with a glass rod properly.
Use a small amount of chemicals for the reaction.
When measuring the weight of the chemical in the conical flask, ensure that the chemical from the ignition tube does not flow in the flask.
Lab Manual Questions
1. What will you learn from the law of conservation of mass experiment?
Ans. We will understand the concept of the law of conservation of mass in a chemical reaction. We can define that the mass of the reactant and the product remains constant during a chemical reaction.
2. What are the precautions taken before doing this experiment?
Ans. Before doing this, we should ensure that the beaker is cleaned properly. The measuring instrument is working properly. Take the weight of the chemical properly to avoid errors in the experiment.
3. What happens when we use Lead nitrate instead of silver nitrate in the reaction with sodium chloride?
Ans. The white precipitate of lead chloride will be formed in place of silver chloride.
Pb (NO3)2+NaCl→PbCl2+NaNO3
4. Define the law of conservation of Mass.
Ans. Antonio Lovosier defines conservation of mass as the total mass of the reactant being equal to the total mass of the product in a chemical reaction. He defines the mass as neither created nor destroyed, it changes its form from one to another.
Viva Questions:
1. What will be the product formed after reacting calcium chloride with sodium sulphate?
Ans. On reacting calcium chloride and sodium sulphate, a white precipitate of calcium sulphate and sodium chloride is formed.
CaCl2 (aq) + Na2SO4 (aq) → CaSO4 (S) + 2NaCl (aq)
2. Is the law of conservation of mass work in burning a candle?
Ans. The law of conservation of mass says that the mass is neither created nor destroyed, it changes its form. In a burning candle, the mass of the candle is converted into a gaseous form in the form of carbon dioxide and water vapour.
3. Who gave the law of conservation of mass?
Ans. The French Scientist Antonio Lavoisier in 1789 gave the concept of the conservation of mass.
4. Is the mass of the burning wood equal to the mass of ashes in a bonfire?
Ans. In a bonfire, the wood burns and is converted into ash, water vapour and gas form. The mass of the wood will be equal to the mass of all three components ash, water vapour and carbon dioxide gas.
5. Define the laws of chemical combination in chemistry.
Ans. In chemistry, two or more elements combine to produce a new compound. This chemical combination of elements involves five laws of chemical combination. All these five laws are known as the law of chemical combination. These are;
Law of conservation of mass
Law of multiple proportions
Law of definite proportion
Gay Lussac’s Law
Avogadro’s law of chemical combination.
6. What is the law of definite proportion?
Ans. In a chemical reaction, the elements are always combined in a specific proportion, irrespective of the quantity present in the reactant. To form a water molecule, hydrogen is taken in two units and oxygen is taken only in one unit.
7. Which chemical reaction does not follow the conservation mass law?
Ans. Nuclear reactions do not follow the conservation of mass, as in this reaction the mass is destroyed and converted into energy.
8. The application of the law of conservation of mass in our daily life?
Ans. The conservation of mass is seen in the combustion of wood, bonfires, the burning of candles, the brewing of beer, the melting of ice, etc.
9. Give some examples of conservation of mass in our ecosystem.
Ans. In our ecosystem we also observe the conservation of mass, plants take carbon dioxide from the atmosphere and water and sunlight and prepare food in the form of glucose. In the photosynthesis process, there is a conservation of mass.
6CO2 + 6H2O → C6H12O6 + 6O2
10. What is the conservation of mass in the decomposition of calcium carbonate?
Ans. When we decompose calcium carbonate, it produces calcium oxide and carbon dioxide. The mass of the calcium carbonate in this reaction will be equal to the mass of calcium oxide and carbon dioxide produced.
Practical Questions:
1. Who gave the concept of the law of multiple proportions?
Julius Robert Mayer
Dmitri Mendeleev
Dalton
Antonio Lavoisier
Ans. The law of multiple proportions was given by Dalton in 1803.
2. The law of conservation of mass states that the mass of the component in a chemical reaction remains.
Constant
Increases
Decreases
None of the above.
Ans. The mass of the component in the reaction remains Constant.
3. When 10 gm of calcium carbonate is decomposed, it produces 4.4 gm of carbon dioxide. How much calcium oxide will produce in this reaction based on the law of conservation of mass?
6.4
5.6
3.4
4.4
Ans. According to the law, the mass of the reactant is equal to the mass of the product. Hence, the Mass of the calcium oxide will be;
Mass of Calcium carbonate = Mass of carbon dioxide + Mass of Calcium oxide
10 gm= 4.4 gm+ X
X= 5.6 gm
4. The law of conservation of mass is also known by which name?
Law of Multiple proportions
Law of chemical Combination
Law of Indestructibility
Law of Conservation of energy.
Ans. Law of indestructibility
5. In a rearrangement reaction, the mass of the reactant and product remains.
The Mass of the Reactant and product remains equal.
The Mass of the Reactant is more than the mass of the product.
The Mass of the product is more than the mass of the reactant.
None
Ans. The mass of the reactant and product remains equal.
6. When does the disinfectant containing hydrogen peroxide come in contact with sunlight?
Hydrogen peroxide decomposes.
Hydrogen peroxide remains constant.
A rearrangement reaction occurs.
An Addition reaction occurs.
Ans. When disinfectant containing hydrogen peroxide comes in contact with sunlight, the hydrogen peroxide decomposes into water and oxygen.
7. What happens when barium chloride is mixed with a solution of sodium sulphate?
White precipitate formed
Brown precipitate formed
Yellow precipitate formed
Black precipitate formed
Ans. A white precipitate of barium sulphate is formed.
8. The Law of conservation of mass is given by which French scientist?
Linus Carl Pauling
Joseph Proust
Gay Lussac
Antonie Laurent Lavoisier
Ans. The law of conservation of mass is given by Antonie Laurent Lavoisier.
9. What is the significance of the conservation of mass?
It is used to calculate the mass of the reactant and product.
It helps in the identification of the product.
It helps in identifying the type of chemical reaction.
It helps in decreasing the efficiency of the reaction.
Ans. It is used to calculate the mass of the reactant and product, and help in the identification of the product, and type of reaction.
10. According to the conservation of mass, when we lit a candle, the mass of the candle is equal to?
Carbon dioxide and water vapour
Water vapour
Carbon dioxide
Light
Ans. Carbon dioxide and water vapour.
Conclusion
From the above experiment, we can verify the conservation of mass in a chemical reaction through experiment. We have found that mass can not be created or destroyed, it changes its form from one state to another state. We have also found that in a chemical reaction, the mass of the reactant and product remains constant.
FAQs on Class 9 Chemistry Law Of Conservation Of Mass Experiment
1. As per the CBSE Class 9 syllabus for 2025-26, what is the law of conservation of mass and why is it a fundamental principle in chemistry?
The law of conservation of mass states that in any isolated system, mass is neither created nor destroyed during a chemical reaction. Therefore, the total mass of the reactants must be equal to the total mass of the products. It is a fundamental principle because it forms the basis of stoichiometry, allowing chemists to quantitatively predict the amounts of substances consumed and produced in reactions.
2. Describe a 5-mark expected experiment to verify the law of conservation of mass using barium chloride and sodium sulphate solutions.
To verify the law of conservation of mass, follow these steps:
- Apparatus: A conical flask, a small test tube (ignition tube), a cork, and a weighing balance.
- Reactants: Prepare a solution of sodium sulphate (Na₂SO₄) and a solution of barium chloride (BaCl₂).
- Procedure:
1. Pour some sodium sulphate solution into the conical flask.
2. Fill the small test tube with barium chloride solution and carefully suspend it in the flask using a thread, ensuring the solutions do not mix.
3. Seal the flask with a cork and weigh the entire apparatus. Record this as the initial mass.
4. Tilt the flask to mix the two solutions. A white precipitate of barium sulphate (BaSO₄) will form.
5. Weigh the flask again after the reaction. Record this as the final mass. - Observation: The initial mass of the sealed flask and its contents is equal to the final mass.
- Conclusion: Since the total mass remains constant, the experiment verifies the law of conservation of mass. The reaction is: BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq).
3. A student heats 12.4 g of copper carbonate (CuCO₃). It decomposes to form copper oxide (CuO) and 4.4 g of carbon dioxide (CO₂). What is the mass of copper oxide formed? Which law is applied here?
This problem is solved using the law of conservation of mass.
The chemical reaction is: CuCO₃(s) → CuO(s) + CO₂(g).
According to the law:
Total Mass of Reactants = Total Mass of Products
Mass of CuCO₃ = Mass of CuO + Mass of CO₂
12.4 g = Mass of CuO + 4.4 g
Mass of CuO = 12.4 g - 4.4 g
Therefore, the mass of copper oxide (CuO) formed is 8.0 g.
4. Why is it a common mistake to think the law of conservation of mass is violated when burning a piece of wood in an open fire?
This is a misconception because the system is not closed. When wood burns, it reacts with oxygen from the air and produces ash, carbon dioxide, and water vapour. Since the gaseous products (CO₂ and H₂O) escape into the atmosphere, the remaining ash weighs less than the original wood, creating the illusion that mass was lost. If the reaction were performed in a sealed container that captured all the gases, the total mass would be conserved.
5. How does Dalton's atomic theory provide a strong theoretical explanation for the law of conservation of mass?
Dalton's atomic theory explains the law of conservation of mass through its core postulate that atoms are indivisible particles that are neither created nor destroyed in a chemical reaction. A reaction is simply a rearrangement of these atoms into new combinations. Since the number and type of atoms remain the same before and after the reaction, their total mass must also remain constant, thus validating the law.
6. For a Class 9 exam, what are some important precautions to take while performing the experiment to verify the law of conservation of mass?
Important precautions for this experiment include:
- The apparatus, such as the conical flask, must be completely sealed with a cork to ensure it is a closed system and no gaseous products can escape.
- The initial weighing must be done only after setting up the entire apparatus, with reactants kept separate inside the sealed flask.
- The reactants should be mixed by gently tilting and swirling the flask, avoiding vigorous shaking that could dislodge the cork.
- The weighing balance should be accurate and placed on a stable, flat surface to avoid errors in measurement.
7. Does the law of conservation of mass apply to nuclear reactions? Explain why or why not from an advanced perspective.
While the law of conservation of mass is strictly valid for all chemical reactions covered in the Class 9 syllabus, it does not strictly apply to nuclear reactions (e.g., fission and fusion). In nuclear reactions, a minute amount of mass is converted into a vast amount of energy, as explained by Albert Einstein's famous equation, E = mc². In this context, it is the total mass-energy that is conserved, not just the mass alone.





































