
Which of the following is steam volatile?
(A) o-Nitrophenol
(B) p-nitrophenol
(C) Both of them
(D) Neither of them
Answer
233.1k+ views
Hint: Any compound that can be distilled with steam distillation process, is called steam volatile compound. One of the compounds from o-nitrophenol and p-nitrophenol forms a special type of bond which lowers its melting and boiling point from the other.
Complete Step-by-Step Solution:
As shown in the hint part, steam volatile compound is a compound that can be distilled by steam distillation process. Now during steam distillation, the compound needs to have its melting point under the boiling point of water so that steam will pass through the solution and the compound will melt if it is in solid state and can be separated from impurities by distillation.
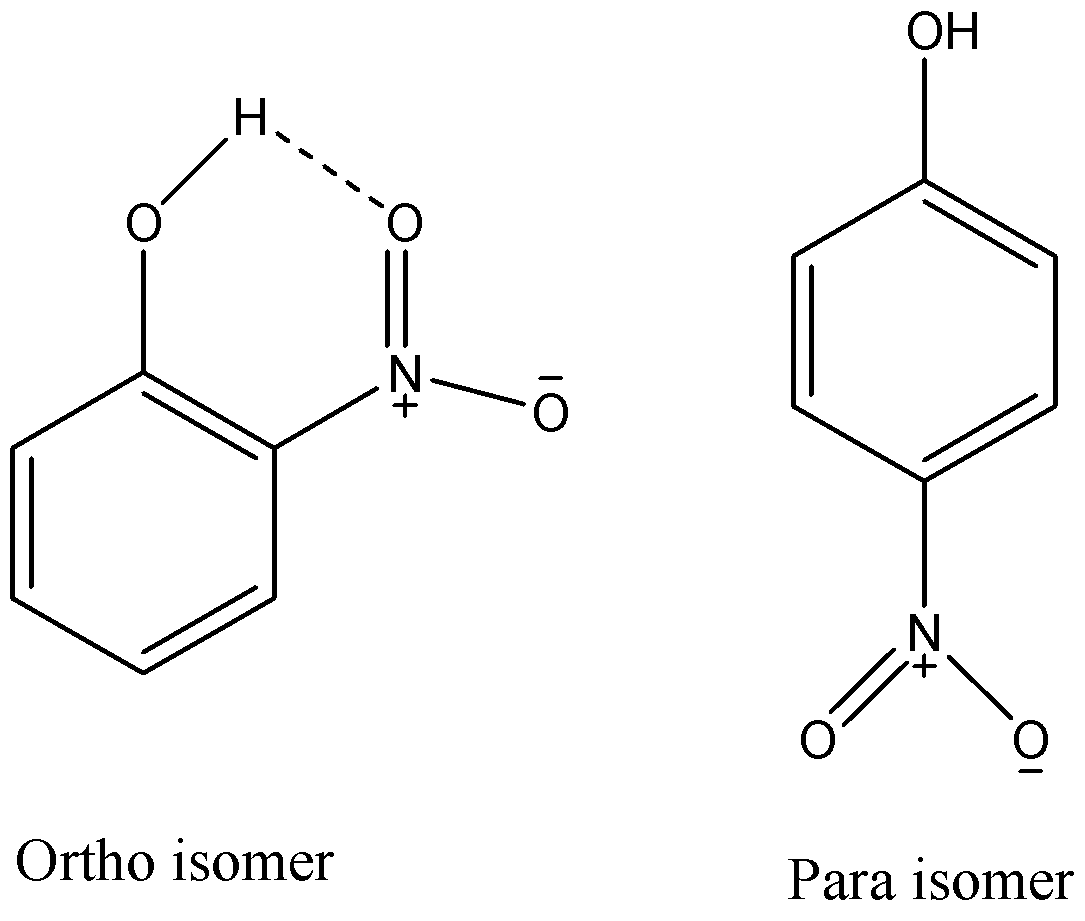
From the above given structures, we can see that ortho isomer has an intramolecular hydrogen bonding while para isomer cannot show it. As hydrogen atoms of o-nitrophenol are involved in intramolecular hydrogen bonding, they cannot effectively form hydrogen bonds with other molecules and hence it will have lower melting and boiling point then the para-isomer.
It is also practically proved that o-nitrophenol has M.P of \[{45^ \circ }C\] and p-nitrophenol has M.P of \[{119^ \circ }C\]. So, as o-nitrophenol has a lower melting point, it will easily melt and get converted to its vapours to get distilled by steam. While p-nitrophenol has a melting point higher than water’s boiling point and hence it will not be distilled with steam distillation. So, we can say that only o-nitrophenol is steam volatile.
Hence correct answer is (A) o-Nitrophenol
Note: Remember that volatile compound and steam volatile compounds are totally different terminologies. Do not get confused with intramolecular and intermolecular hydrogen bonding as both are different and have different effects on the melting point of a compound.
Complete Step-by-Step Solution:
As shown in the hint part, steam volatile compound is a compound that can be distilled by steam distillation process. Now during steam distillation, the compound needs to have its melting point under the boiling point of water so that steam will pass through the solution and the compound will melt if it is in solid state and can be separated from impurities by distillation.

From the above given structures, we can see that ortho isomer has an intramolecular hydrogen bonding while para isomer cannot show it. As hydrogen atoms of o-nitrophenol are involved in intramolecular hydrogen bonding, they cannot effectively form hydrogen bonds with other molecules and hence it will have lower melting and boiling point then the para-isomer.
It is also practically proved that o-nitrophenol has M.P of \[{45^ \circ }C\] and p-nitrophenol has M.P of \[{119^ \circ }C\]. So, as o-nitrophenol has a lower melting point, it will easily melt and get converted to its vapours to get distilled by steam. While p-nitrophenol has a melting point higher than water’s boiling point and hence it will not be distilled with steam distillation. So, we can say that only o-nitrophenol is steam volatile.
Hence correct answer is (A) o-Nitrophenol
Note: Remember that volatile compound and steam volatile compounds are totally different terminologies. Do not get confused with intramolecular and intermolecular hydrogen bonding as both are different and have different effects on the melting point of a compound.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




