
Identify which of the above shown graphs represent the force between two electrons Vs distance between them?
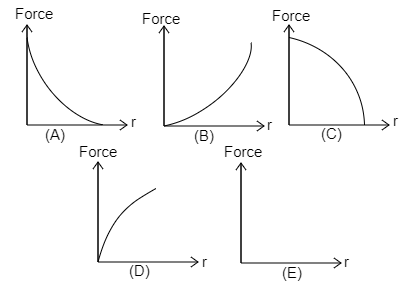
(A) Graph A
(B) Graph B
(C) Graph C
(D) Graph D
(E) Graph E
Answer
241.2k+ views
Hint: The force between the electrons can be determined from the Coulomb’s law of electric charges. This law is also called the inverse square law. Where, the force is inversely proportional to the square of the distance. And the force is also called the electrostatic force.
Complete Step by step solution:
The Coulomb’s law of attraction or repulsion states that the force between two charges will be proportional to the product of magnitude of two charges and inversely proportional to the square of the distance between them.
$F = \dfrac{1}{{4\pi {\varepsilon _0}}}\dfrac{{{q_1}{q_2}}}{{{r^2}}}$
Where, ${q_1}$ and ${q_2}$ are the magnitude of two charge, $r$ is the distance between the two charges and ${\varepsilon _0}$ is the permittivity of free space.
For the force between the two electrons of charge $e$,
$\Rightarrow F = \dfrac{1}{{4\pi {\varepsilon _0}}}\dfrac{{e \times e}}{{{r^2}}}$
It is clear that the force is inversely proportional to the square of the distance between them. When the electrons are far apart, then the force between them will be much smaller. Thus, the force of repulsion is less when they are far. When the distance is small that is if they are near the force of repulsion will be greater. The decrease is not linear. The decrease in force with increase in distance will be exponentially. Hence the graph A shows the decrease in force $F$ with increase in distance $r$.
The answer is option A.
Note: The force is represented in the Y- axis and the distance between the electrons in X- axis where the small increase in distance will bring large decrease in force. Hence, they related to inverse square law. The force is directly proportional to the product of the two charges.
Complete Step by step solution:
The Coulomb’s law of attraction or repulsion states that the force between two charges will be proportional to the product of magnitude of two charges and inversely proportional to the square of the distance between them.
$F = \dfrac{1}{{4\pi {\varepsilon _0}}}\dfrac{{{q_1}{q_2}}}{{{r^2}}}$
Where, ${q_1}$ and ${q_2}$ are the magnitude of two charge, $r$ is the distance between the two charges and ${\varepsilon _0}$ is the permittivity of free space.
For the force between the two electrons of charge $e$,
$\Rightarrow F = \dfrac{1}{{4\pi {\varepsilon _0}}}\dfrac{{e \times e}}{{{r^2}}}$
It is clear that the force is inversely proportional to the square of the distance between them. When the electrons are far apart, then the force between them will be much smaller. Thus, the force of repulsion is less when they are far. When the distance is small that is if they are near the force of repulsion will be greater. The decrease is not linear. The decrease in force with increase in distance will be exponentially. Hence the graph A shows the decrease in force $F$ with increase in distance $r$.
The answer is option A.
Note: The force is represented in the Y- axis and the distance between the electrons in X- axis where the small increase in distance will bring large decrease in force. Hence, they related to inverse square law. The force is directly proportional to the product of the two charges.
Recently Updated Pages
JEE Main 2025-26 Mock Tests: Free Practice Papers & Solutions

JEE Main 2025-26 Experimental Skills Mock Test – Free Practice

JEE Main 2025-26 Electronic Devices Mock Test: Free Practice Online

JEE Main 2025-26 Atoms and Nuclei Mock Test – Free Practice Online

JEE Main 2025-26: Magnetic Effects of Current & Magnetism Mock Test

JEE Main Mock Test 2025: Properties of Solids and Liquids

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Physics Question Paper 2026: Download SET-wise PDF with Answer Key & Analysis

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Class 12 Physics Question Paper Set 3 (55/1/3) 2025 – PDF, Solutions & Analysis

CBSE Class 12 Physics Set 2 (55/2/2) 2025 Question Paper & Solutions

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26




