
Haloform reaction with ${{I}_{2}}$ and $KOH$ will be responded by:
A. 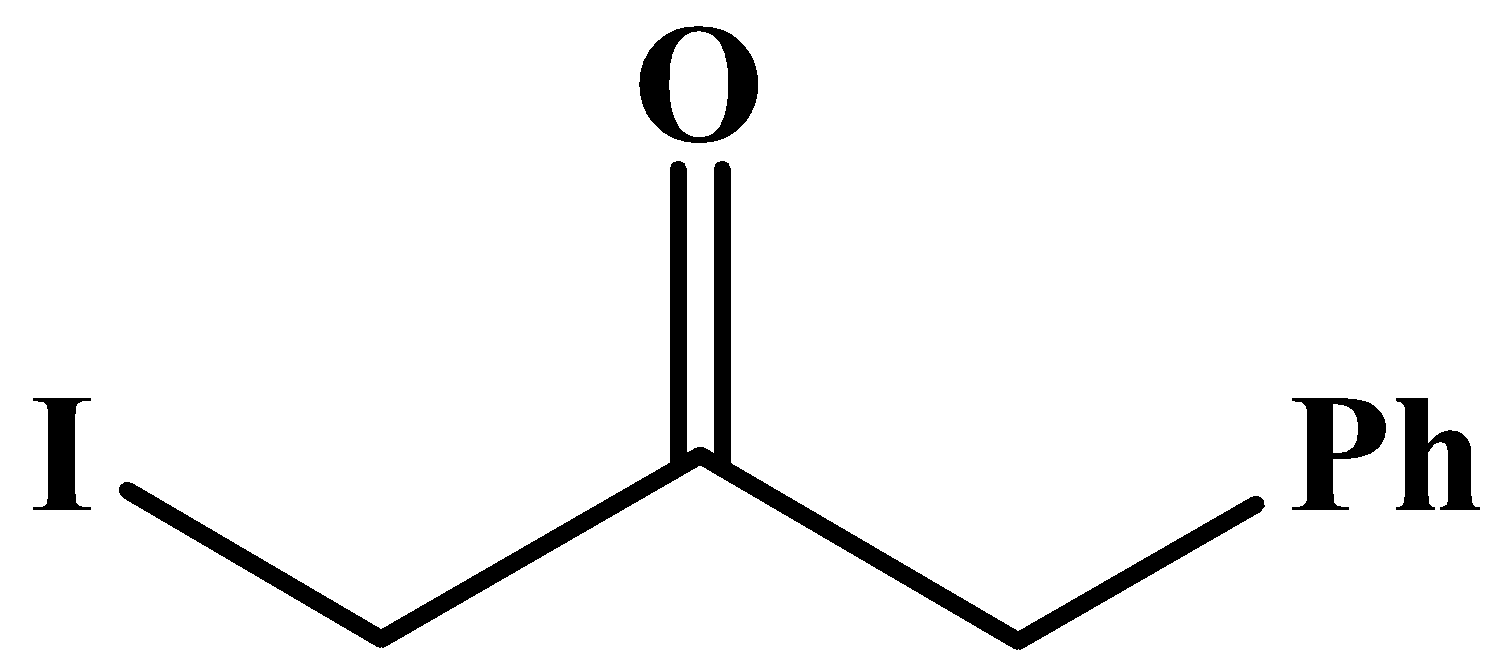
B. 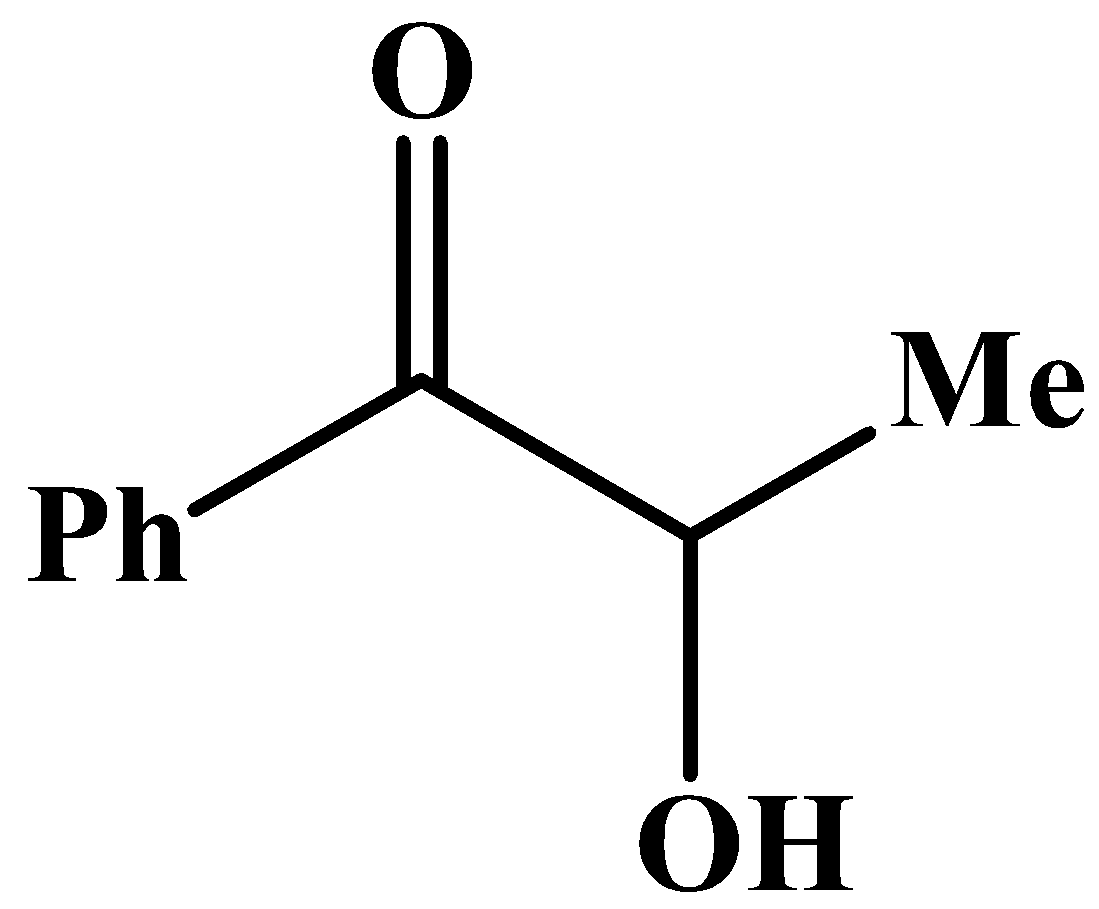
C. 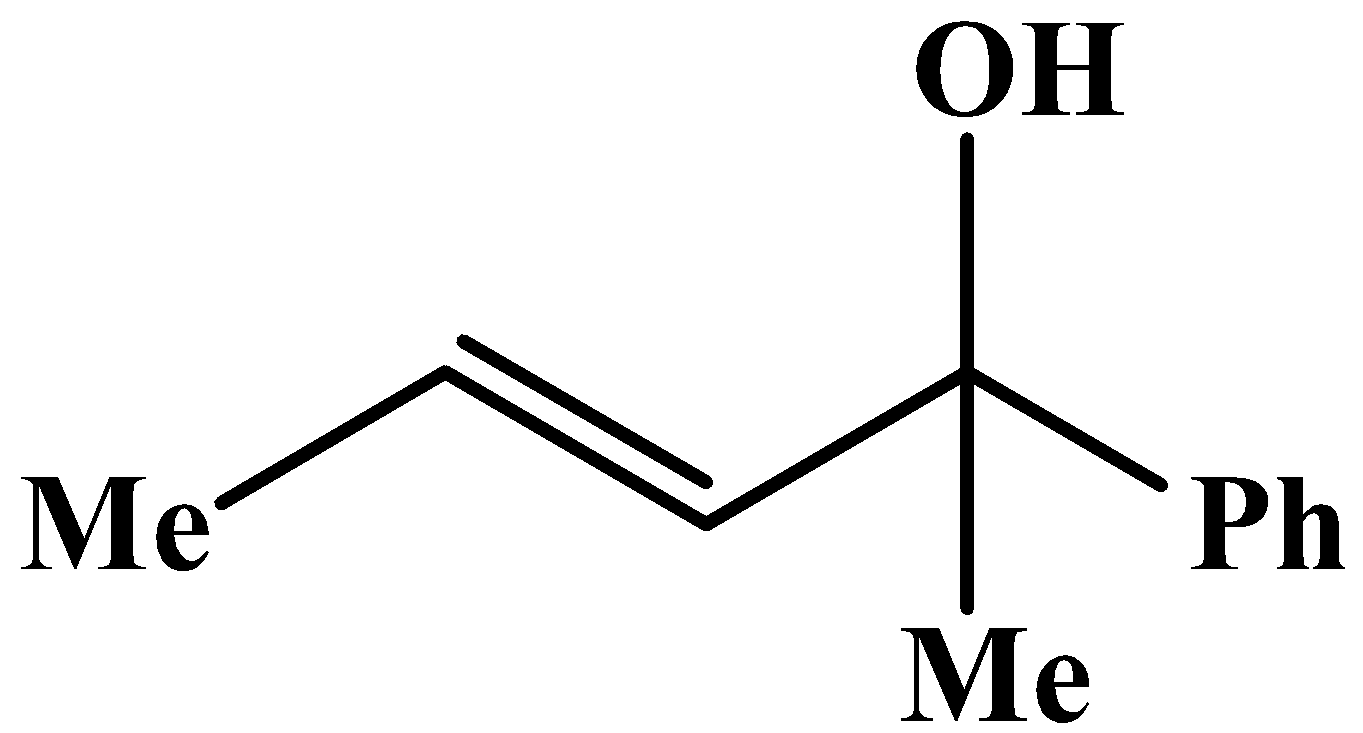
D. 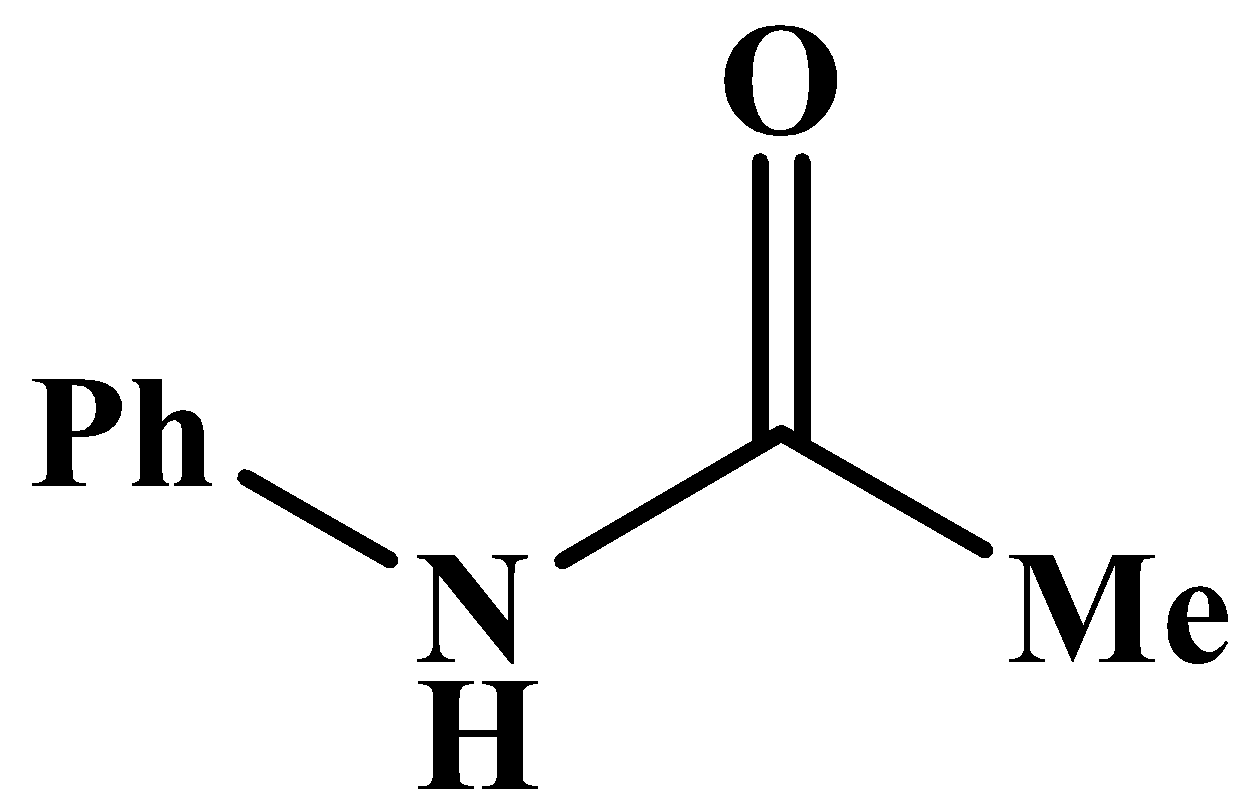
Answer
233.1k+ views
Hint: Haloform reaction is oxidising in nature. It requires a methyl group near a carbonyl carbon to give a positive test for this reaction.
Complete step-by-step answer:
The Haloform reaction is actually used to distinguish aldehydes and ketones which have a methyl group attached to the carbonyl carbon from the rest of the mixture. This reaction is oxidising in nature and therefore its products are a carboxylic acid which has one carbon atom less and a methyl halide. This is the same methyl group that was attached to the alpha carbon atom (the carbon atom bearing the carbonyl oxygen). The reaction is shown as below:
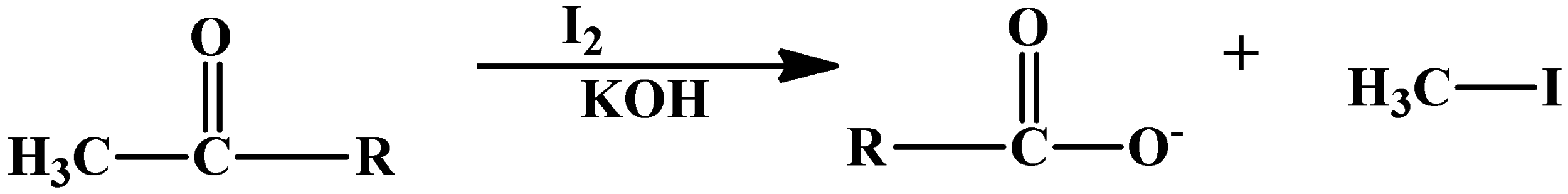
The “R” group here can be a hydrogen atom, making it an aldehyde or can be another alkyl group making it a ketone. This reaction does not affect other double bonds. So in a way the aromatic compounds can also undergo this process.
This reaction can also happen with alcohols, because they oxidise to carbonyl compounds. An example with ethyl alcohol is as below:
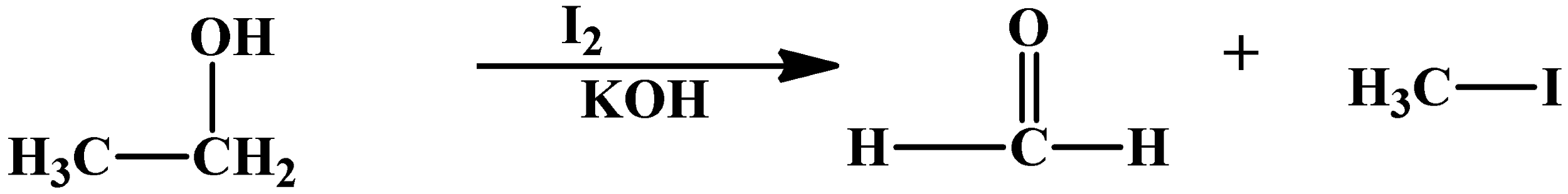
As you can see, ethyl alcohol loses a methyl group and then oxidises into formaldehyde. The by product is same as any Haloform reaction, which is a methyl halide.
Let’s look into the options that are given one-by-one:
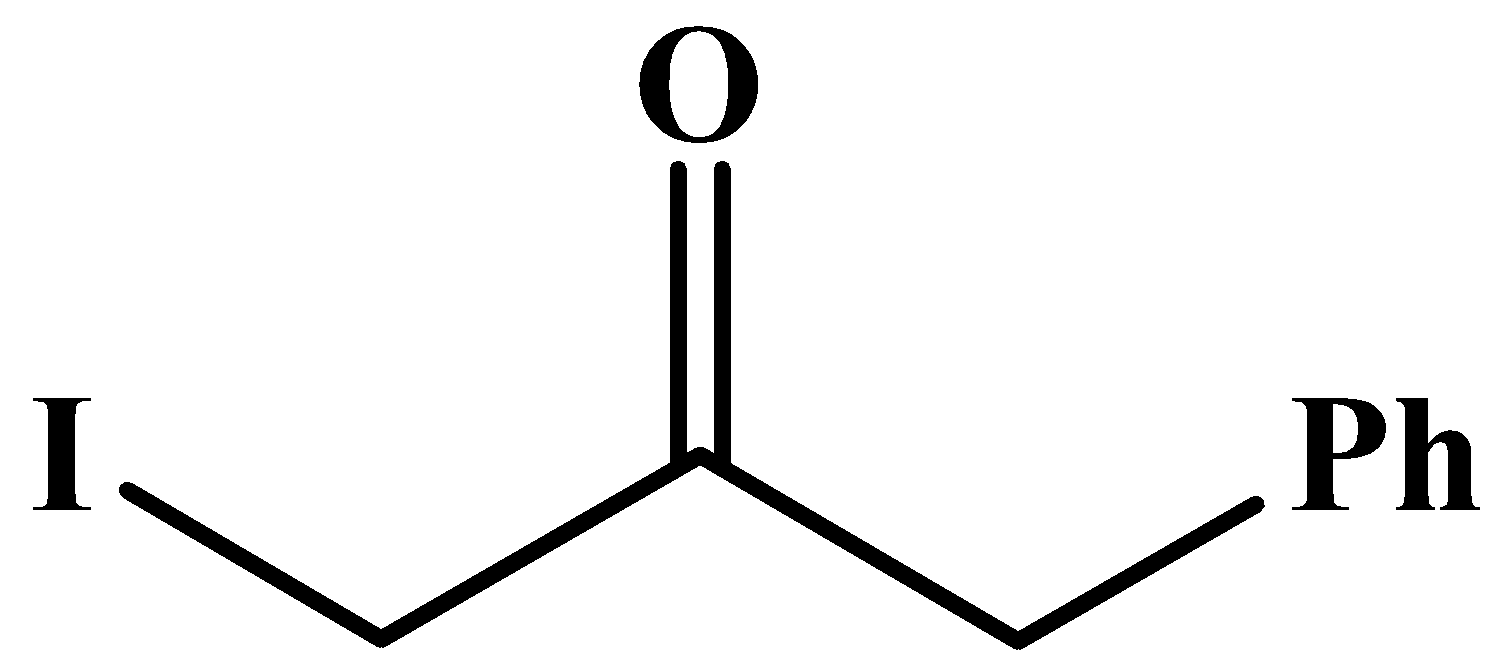
The carbonyl group here is in the middle of the compound and it has no methyl groups attached to it. Methyl groups are only possible at any end of a molecular chain or at the end of a branched chain.
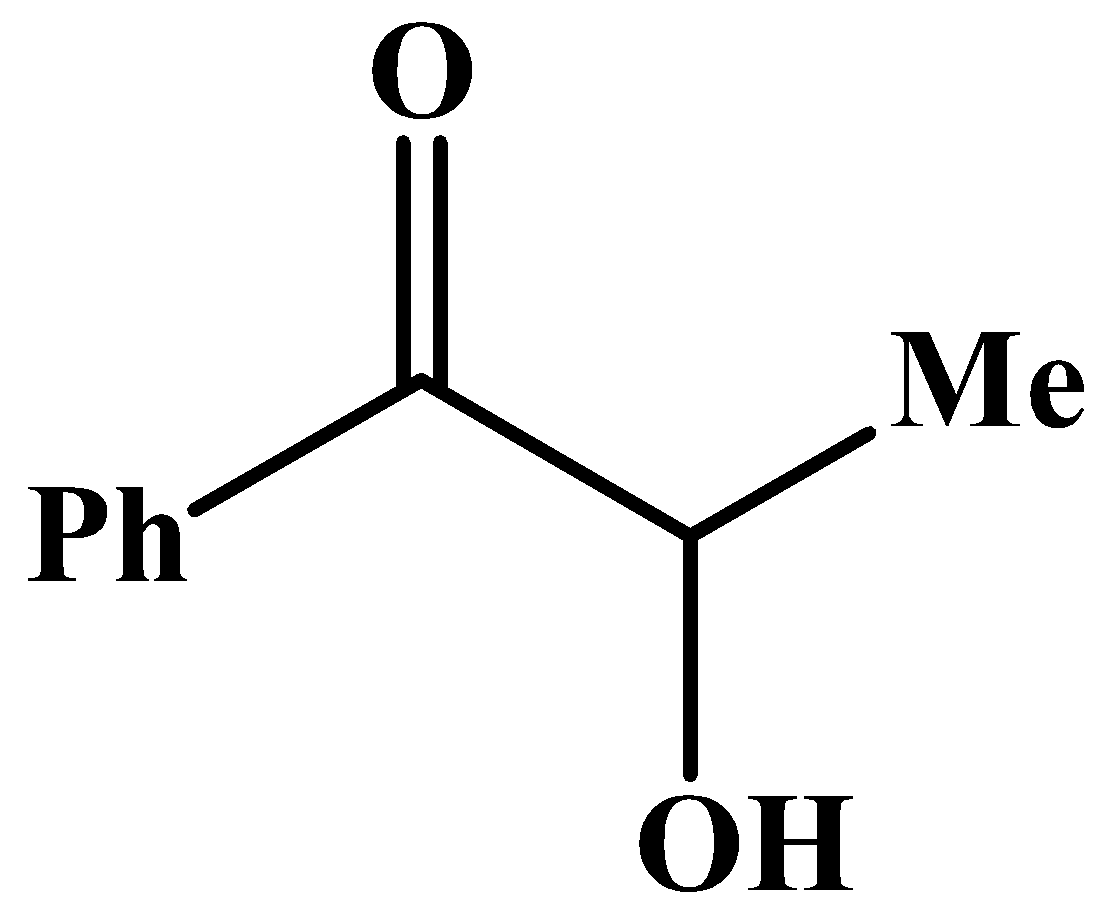
The carbonyl group has a phenyl group at its side and no other methyl group.
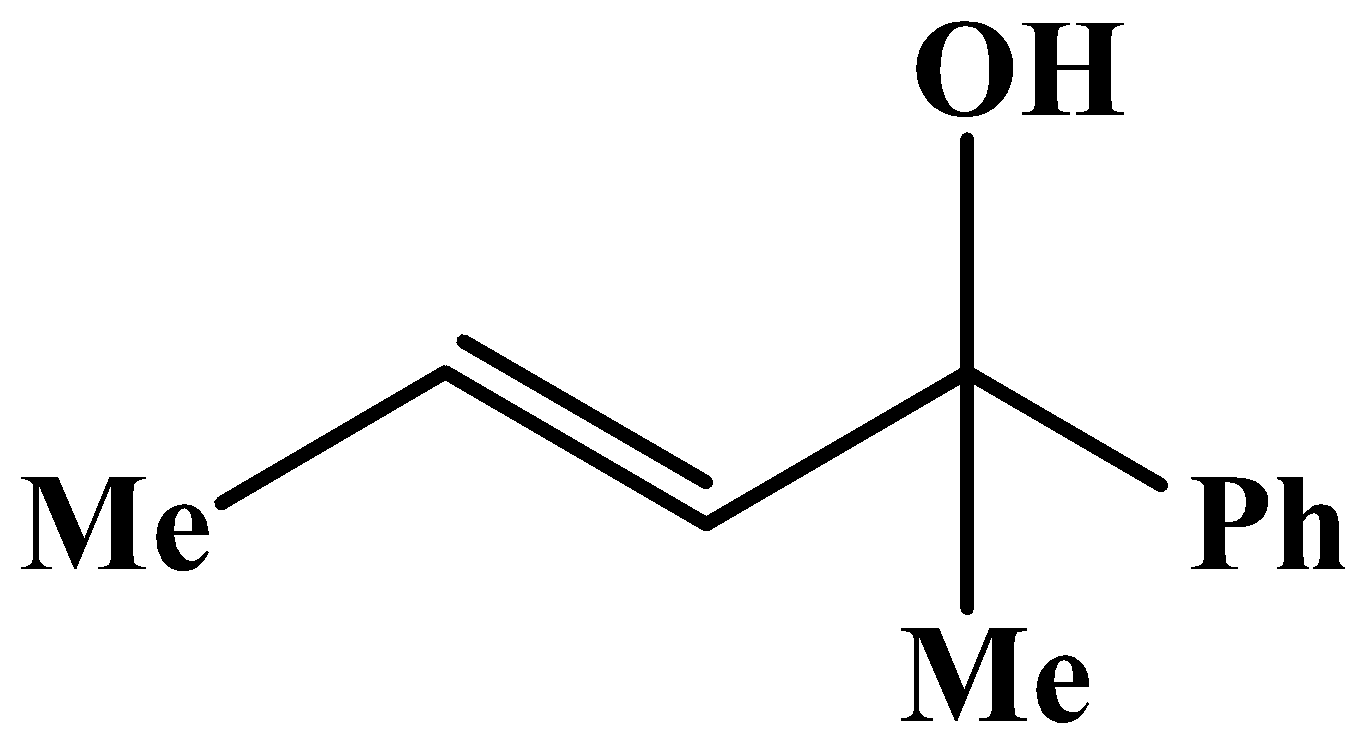
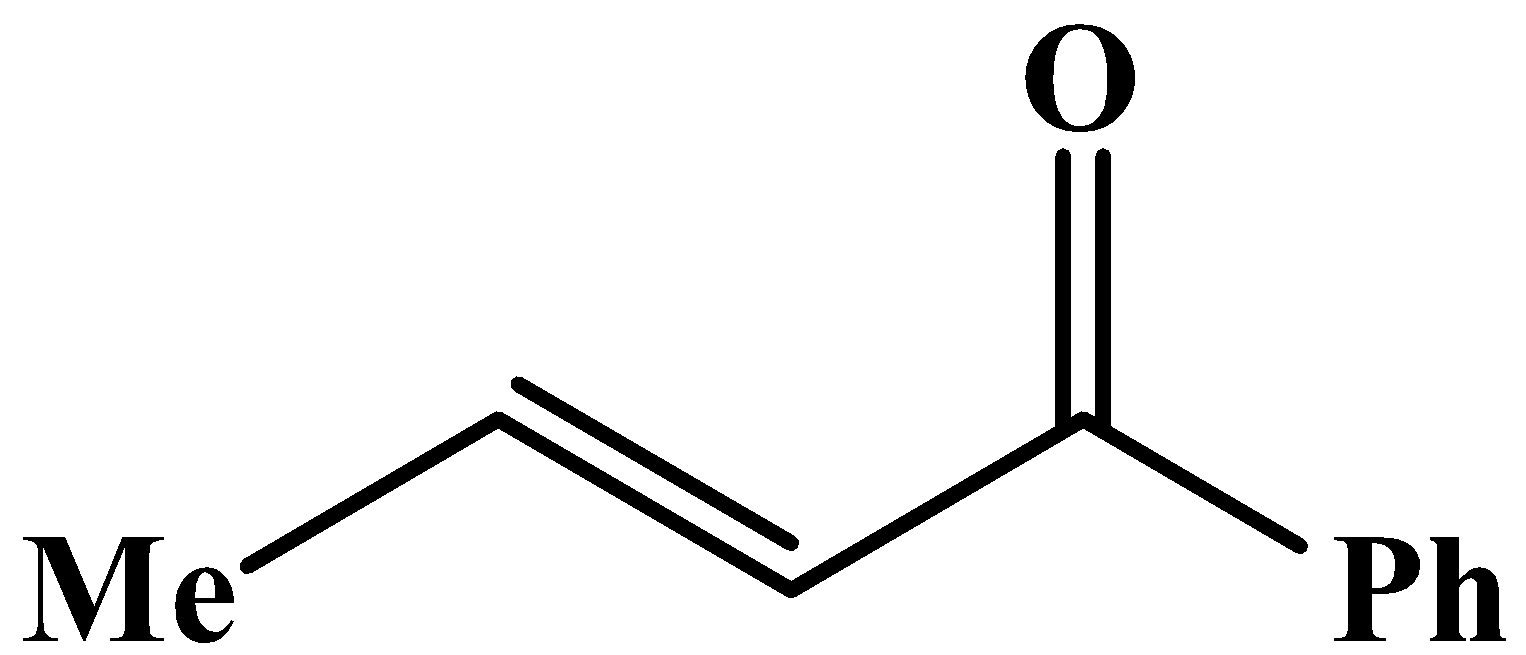
The methyl group is attached to the carbon bearing the hydroxyl group. This will respond to the oxidation reaction because it will convert into its corresponding carbonyl compound, which will be as shown below:
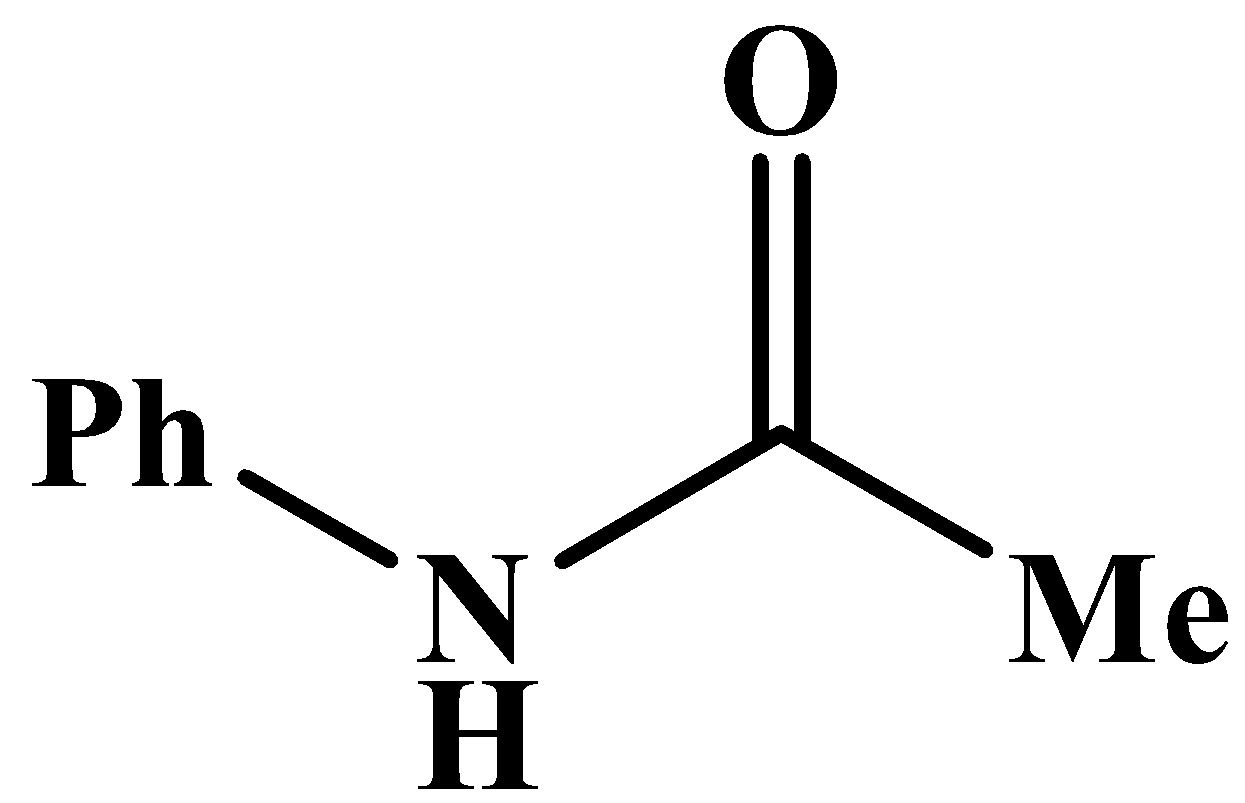
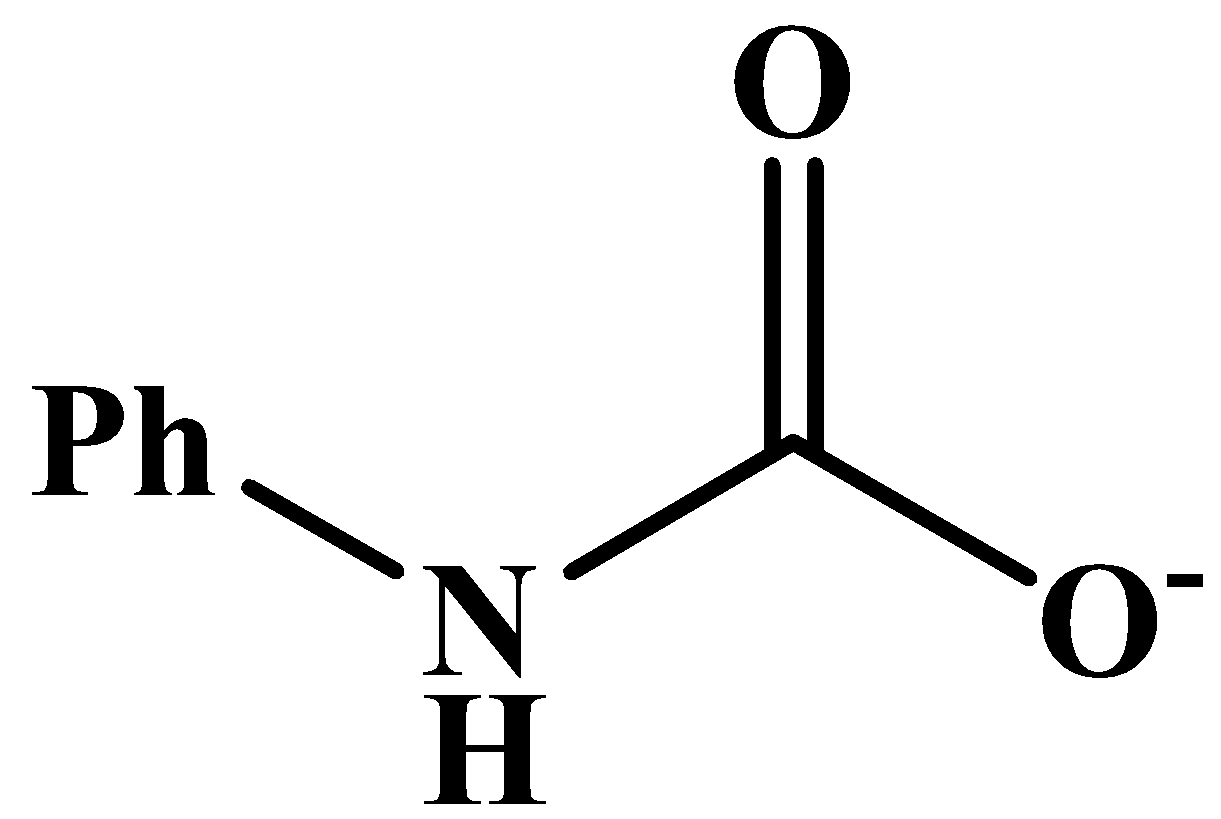
This will also respond to the given Haloform reaction as the methyl group is directly attached to the carbonyl carbon. The product of the reaction is:
Note:
The products of this reaction cannot be converted back into their original forms. This is because the compound that undergoes this reaction loses a methyl group, hence becoming shorter. Therefore this reaction is not used to oxidise carbonyl compounds into carboxylic acids or alcohols into aldehydes or ketones. This is only a reaction that can detect the presence of certain specific compounds. The by-products of this reaction are also a nuisance to separate.
Complete step-by-step answer:
The Haloform reaction is actually used to distinguish aldehydes and ketones which have a methyl group attached to the carbonyl carbon from the rest of the mixture. This reaction is oxidising in nature and therefore its products are a carboxylic acid which has one carbon atom less and a methyl halide. This is the same methyl group that was attached to the alpha carbon atom (the carbon atom bearing the carbonyl oxygen). The reaction is shown as below:

The “R” group here can be a hydrogen atom, making it an aldehyde or can be another alkyl group making it a ketone. This reaction does not affect other double bonds. So in a way the aromatic compounds can also undergo this process.
This reaction can also happen with alcohols, because they oxidise to carbonyl compounds. An example with ethyl alcohol is as below:

As you can see, ethyl alcohol loses a methyl group and then oxidises into formaldehyde. The by product is same as any Haloform reaction, which is a methyl halide.
Let’s look into the options that are given one-by-one:

The carbonyl group here is in the middle of the compound and it has no methyl groups attached to it. Methyl groups are only possible at any end of a molecular chain or at the end of a branched chain.

The carbonyl group has a phenyl group at its side and no other methyl group.

The methyl group is attached to the carbon bearing the hydroxyl group. This will respond to the oxidation reaction because it will convert into its corresponding carbonyl compound, which will be as shown below:


This will also respond to the given Haloform reaction as the methyl group is directly attached to the carbonyl carbon. The product of the reaction is:
Note:
The products of this reaction cannot be converted back into their original forms. This is because the compound that undergoes this reaction loses a methyl group, hence becoming shorter. Therefore this reaction is not used to oxidise carbonyl compounds into carboxylic acids or alcohols into aldehydes or ketones. This is only a reaction that can detect the presence of certain specific compounds. The by-products of this reaction are also a nuisance to separate.

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




