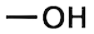An Introduction to IUPAC Nomenclature
IUPAC nomenclature of organic compounds refers to the systematic approach taken for the nomenclature of organic compounds as per the recommendation of the International Union of Pure and Applied Chemistry.
There was a necessity for such a systematic approach that arose due to the large quantity of new discoveries of organic compounds, which made the trivial nomenclature of organic compounds highly inconvenient. But still, the IUPAC nomenclature guidelines are not always followed by chemists since some compounds have very long and extremely tedious names as per the IUPAC nomenclature guidelines. These compounds are assigned more insignificant names.
IUPAC Nomenclature: Organic Chemistry
The organic chemistry nomenclature IUPAC is a method of naming organic chemical compounds recommended by the International Union of Pure and Applied Chemistry (IUPAC). It has been included in the Organic Chemistry Nomenclature. Ideally, every organic compound should have a name from which an unmistakable structural formula can be constructed. There is also an IUPAC nomenclature for inorganic chemistry.
To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when a compound requires an unambiguous and absolute definition. IUPAC names are sometimes simpler than older names, such as ethanol rather than ethyl alcohol.
Salient Features of the IUPAC System
The IUPAC system is the most widely used method for naming organic compounds. The key features of this system are as follows:
The first step in naming an organic compound using the IUPAC system is to determine its structure. This can be done using various methods, such as drawing its structural formula or using a molecular model kit.
Once the structure of the compound has been determined, the next step is to identify the longest continuous chain of carbon atoms in the molecule. This chain is known as the 'parent chain'.
The parent chain is then given a base name, which depends on the number of carbons it contains. For example, a parent chain with four carbons would be called 'butane'.
Functional groups are then identified and named according to their chemical properties. For example, a compound with a hydroxyl group (OH) would be called alcohol.
Finally, the location of each functional group on the parent chain is indicated by a number. For example, if there are two OH groups on a butane molecule, they would be numbered 1- and 2-butanol, respectively.
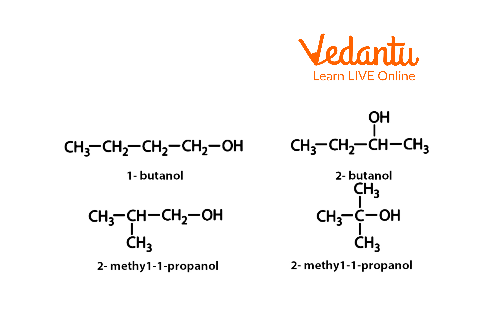
Examples of Alcohol
Rule for Naming
The IUPAC nomenclature system is a set of rules that chemists use to name chemical compounds. The rules are designed to give each compound a unique and unambiguous name.
There are five main rules for naming organic compounds using the IUPAC system:
Find the longest continuous chain of carbon atoms in the compound. This is the "parent chain".
Identify the functional groups present in the compound and determine their highest priority.
Based on the parent chain and functional groups, determine the suffix of the compound (-ane, -ene, -yne, etc.).
Number the carbons in the parent chain so that the functional group with the lowest number has the lowest priority.
Name each substituent group by its appropriate prefix (methyl-, ethyl-, propyl-, etc.) and indicate its position on the parent chain with a number preceded by a dash (-).
IUPAC Nomenclature Methods
The IUPAC nomenclature system is a set of rules that chemists use to name chemical compounds. The system is based on the International Union of Pure and Applied Chemistry (IUPAC) recommendations.
There are five main types of IUPAC names:
Systematic names
Semi-systematic names
Common names
Trade names
Trivial names
Systematic Names: Systematic names are generated by using the chemical structure of the compound to determine its base name. The base name is then modified by adding prefixes and suffixes that describe the functional groups present in the molecule.
For example, the systematic name for ethanol would be ethane-1,2-diol because it contains two carbon atoms (the “eth” part of the name), it has a single bond between those carbons (the “ane” part of the name), and there are two hydroxyl groups (-OH) attached to each carbon atom (the “diol” part of the name).
Semi-Systematic Names: Semi-systematic names are similar to systematic names, but they use common abbreviations for some functional groups instead of including them in the base name.
Common Names: A common name is defined by the IUPAC as a name that unambiguously defines a chemical, yet does not follow the current systematic naming convention. An example of a common name is acetone, which has the systematic name 2-propanone.
Trade Names: Trade names is a public-facing name used by a company to identify itself or to identify a product or substance that it either sells or utilises internally. They are different from the legal names given to a corporation or the scientific names associated with a product or substance.
It’s important to note that trade names are not regulated by IUPAC; companies can choose any trade name they want as long as it isn’t already being used by another company .
Trivial Names: Trivial Names are informally assigned to compounds, and they don’t necessarily follow any naming conventions . For example, ethanol was originally given the trivial name “spiritus frumenti” because it was produced by distilling grain spirits . Other examples of trivial names include formaldehyde, which was originally named “methanal”; acetic acid, which was originally named “vinegar acid".
The IUPAC nomenclature of aliphatic compounds is based on the name of the longest chain of carbon atoms in the molecule. The chain is numbered from one end to the other, and the position of each substituent is indicated by its number. The suffix "-ane" is added to indicate that all carbons are saturated (i.e., single-bonded).
If there are two or more substituents on a given carbon atom, they are listed in alphabetical order and separated by commas. If there are three or more substituents, they are again listed in alphabetical order but separated by hyphens. When more than one possible numbering system exists for a given compound, the lowest numbers are always used.
The prefix "di-" indicates two identical groups, "tri-" indicates three identical groups, etc. The prefixes "bis-," "tris-," etc., may also be used instead of "di-," "tri-," etc., when it is necessary to avoid ambiguity (for example, when there might be confusion about which atoms constitute a particular group).
The term 'alkane' refers to a saturated hydrocarbon, that is an organic compound consisting only of hydrogen and carbon atoms bonded together by single covalent bonds.
Alkanes have the general chemical formula \[{{C}_{n}}{{H}_{2n+2}}\], where n is an integer greater than 0.
The simplest alkane is methane $(C{{H}_{4}})$, which has one carbon atom bonded to four hydrogen atoms (thus n=1).
Ethane \[({{C}_{2}}{{H}_{6}})\] has two carbons bonded together with single bonds.
Propane \[({{C}_{3}}{{H}_{8}})\] has three carbons bonded together with single bonds,
Butane \[({{C}_{4}}{{H}_{10}})\] has four carbons bonded together with single bonds,
Pentane \[({{C}_{5}}{{H}_{12}})\] has five carbons bonded together with single bonds;
Hexane \[({{C}_{6}}{{H}_{14}})\] has six carbons bonded together with single bonds;
Heptane \[({{C}_{7}}{{H}_{16}})\] has seven carbons bonded together with single bonds;
Octane \[({{C}_{8}}{{H}_{18}})\] has eight carbons bonded together with single bonds;
Nonane \[({{C}_{9}}{{H}_{20}})\] has nine carbons bonded together with single bonds;
Decane \[({{C}_{9}}{{H}_{20}})\] has ten carbons bonded together with single bonds and so on.
Thus, the general formula for alkanes is \[{{C}_{n}}{{H}_{2n+2}}\]where n is any post integer greater than zero.
List of Functional Groups in Organic Chemistry
The following is the table of the common functional groups you will encounter in organic chemistry:
Summary
IUPAC nomenclature of organic compounds refers to the systematic approach taken for the nomenclature of organic compounds as per the recommendation of the International Union of Pure and Applied Chemistry (often abbreviated to IUPAC). Here, we have discussed the salient features of IUPAC nomenclature and a few nomenclature methods.
FAQs on IUPAC Nomenclature Of Organic Compounds - NEET Important Topic
1. What is a trivial nomenclature system?
A trivial nomenclature system involves a non-systematic approach while naming any compound. Typically, the terms used every day to describe an organic or inorganic substance, are registered as its official name. Therefore, compounds that have derived their name via this system have a much simpler name compared to others.
Trivial nomenclature is a system of naming compounds based on their common names, which might be derived from the origin, a place, or even at random. HCOOH is known as formic acid because it is a molecule found in ants. In Latin, ants are called Formica. As a result of this system, it was possible for different people to give the same compound different names.
2. Why is the nomenclature of organic compounds important?
The purpose of the IUPAC system of nomenclature is to establish an international standard of naming compounds to facilitate communication. The goal of the system is to give each structure a unique and unambiguous name, and to correlate each name with a unique and unambiguous structure. This means that each chemical name should refer to a single substance and has a different name on the basis of its structure.
3. What are the drawbacks of the trivial nomenclature system?
Some drawbacks of the trivial nomenclature system are as follows:
A few shortcomings of the trivial system for naming organic compounds are listed below.
Several trivial names can exist for one specific compound. An example of this can be observed in the alternate names of phenol, for which names such as hydroxybenzene and carbolic acid also exist.
The trivial nomenclature system is limited to only a few compounds in each specific group. An example of this is: the first two members belonging to the carboxylic acid group have the trivial names formic acid and acetic acid. However, no trivial names exist for carboxylic acids with a greater number of atoms.
There exist no particular set of guidelines for the nomenclature of complex compounds in the trivial system.