Revision Notes on The P-Block Elements for NEET 2026 - Free PDF Download
p-Block Elements is a really important chapter for students who are in class 11. They will get to learn about the different elements that are known as P Block Elements. These elements are the ones that are included on the right side of the Periodic table. Some common examples of P Block elements include Nitrogen, Oxygen, Fluorine, as well as noble gases excluding Helium. For a better understanding of the chapter, students are advised to download P Block Elements Class 11 notes.
The Revision notes are incredible study materials that have been developed by learned subject matter experts at Vedantu. Included in the revision notes are all the necessary information and concepts of the chapter that can help students in their NEET preparation. Studying from the P-Block Elements notes will enable students to grasp the chapter concept very easily.
Access NEET Revision Notes Chemistry - The p-Block Elements
The p-Block Elements
p-block elements are those elements for which the last electron enters the outermost p-orbital.
As there are three p-orbitals and can occupy electrons maximum up to number six, therefore, there are 6 groups of p-block elements in the modern periodic table from group-13 to group-18.
All the p-block elements, the valence shell electronic configuration is $n{s^2}n{p^{1 - 6}}$ except for helium.
p-block elements consist of both metalloids and non-metal elements of the periodic table.
Group-13 Elements
The Boron Family
Group-13 elements show variation in their occurrence and properties.
Boron, the first member of Group-13 has slight metalloid character while aluminium is a typical metal but shows chemical similarities to boron, gallium, indium and thallium.
Electronic Configuration
The valance shell electronic configuration of these elements is $n{s^2}n{p^1}$.
Electronic configuration of elements of group-13 are as follows:
$B - \left[ {He} \right]2{s^2}2{p^1}$
$Al - \left[ {Ne} \right]3{s^2}3{p^1}$
$Ga - \left[ {Ar} \right]3{d^{10}}4{s^2}4{p^1}$
$In - \left[ {Kr} \right]4{d^{10}}5{s^2}5{p^1}$
$Tl - \left[ {Xe} \right]4{f^{14}}5{d^{10}}6{s^2}6{p^1}$
Atomic Radii
Generally, on moving down the group the number of shells increases therefore, atomic radii also increase.
An exception is seen as the atomic radius of gallium is less than that of aluminium.
The presence of d-electrons in gallium provides a poor screening effect for the valance shell electron and as a result, effective nuclear charge in gallium increases and tightly holds the electrons therefore the size of the atom shrinks.
Ionisation Enthalpy
The ionisation enthalpy of the boron family is very unsystematic and does not decrease smoothly down the group.
Firstly, there is a decrease from Boron to Aluminium because of an increase in size. There is a discontinuity in ionisation enthalpy values between aluminium and gallium, and between indium and thallium.
This is due to the inability of d and f-electrons to screen well as this less screening effect compensates for the increase in effective nuclear charge as a result ionisation enthalpy increases.
Electronegativity:
As we move down the group electronegativity first decreases from Boron to Aluminium and then an increase is observed.
This unsystematic order is because of the discrepancies in the atomic size of the elements.
Physical Properties
Boron is a solid, hard, and black in colour.
Due to its very high crystalline lattice, boron has a high melting point while the rest of the members are soft metals, and have a low melting point and high electrical conductivity.
Out of all, gallium has the lowest melting point of $303K$ and can exist in liquid form during summer. The density of elements increases from boron to thallium.
Chemical Properties:
Oxidation State:
As we move down the group, the sum of the first three ionisation enthalpies decreases and as a result metals are able to form $+ 3$ state.
Gallium, Indium, and Thallium show both $+ 1$ and $+ 3$ oxidation states, but the stability of $+ 1$ oxidation state increases for heavier metals.
$Al < Ga < In < Tl$
Reactivity Towards Air:
In crystalline form, $B$ is unreactive and aluminium forms a thin layer of its oxide on its surface.
Elements of group 13 react with oxygen and nitrogen in air (on heating) and form oxides and nitrides respectively.
The Reaction is as Follows:
$2E\left( s \right) + 3{O_2}\left( g \right)\xrightarrow{\Delta }2{E_2}{O_3}\left( s \right)$
$2E\left( s \right) + {N_2}\left( g \right)\xrightarrow{\Delta }2EN\left( s \right)$
The oxides form varies in properties. Boron trioxide is acidic in nature, aluminium trioxide is amphoteric while the oxides of gallium, indium and thallium are basic in nature.
Reactivity Towards Acids and Alkalis:
Boron is unreactive towards acids and alkalis.
Aluminium reacts with both mineral acids and aqueous alkalis this shows a amphoteric character.
Reaction of aluminium with acid:
$2Al\left( s \right) + 6HCl\left( {aq} \right)\xrightarrow{{}}2A{l^{3 + }}\left( {aq} \right) + 6C{l^ - }\left( {aq} \right) + 3{H_2}\left( g \right)$
Reaction of aluminium with alkali:
$2Al\left( s \right) + 2NaOH\left( {aq} \right) + 6{H_2}O\left( l \right)\xrightarrow{{}}2N{a^ + }{\left[ {Al{{\left( {OH} \right)}_4}} \right]^ - }\left( {aq} \right) + 3{H_2}\left( g \right)$
Reactivity Towards Halogens:
In reaction with halogens, boron family elements form trihalides.
$2E\left( s \right) + 3{X_2}\left( g \right)\xrightarrow{{}}2E{X_3}\left( s \right)$
Anomalous Trends in Properties of Boron:
The trichlorides, bromides, iodides of all elements are covalent in nature. There are certain complexes like ${\left[ {M{{\left( {OH} \right)}_4}} \right]^ - }$and ${\left[ {M{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$ which exists for all elements except boron.
The monomer of trihalides are electron deficiency species and act as Lewis acids.
The boron tri-fluoride reacts readily with ammonia (Lewis base) to complete its octet.
$B{F_3} + :N{H_3}\xrightarrow{{}}{F_3}B \leftarrow N{H_3}$
Boron doesn’t have d-orbital to expand its octet like other elements of group; thus its maximum covalence is four.
Some Important Compounds of Boron:
Borax
It’s the white crystalline solid that is soluble in water to give an alkaline solution.
Chemical formula for borax is: $N{a_2}{B_4}{O_7}.10{H_2}O$
On heating, borax loses its water molecule and swells up.
On further heating, it changes into a transparent liquid which is solidified into glass-like material called a borax bead.
$N{a_2}{B_4}{O_7}.10{H_2}O\xrightarrow{\Delta }N{a_2}{B_4}{O_7}\xrightarrow{\Delta }2NaB{O_2} + {B_2}{O_3}$
Orthoboric Acid
It is a white crystalline solid with soapy touch which is sparingly soluble in water.
It is prepared by the reaction of an acid with an aqueous solution of borax.
The Reaction of the Above Statement is as Follows:
$N{a_2}{B_4}{O_7} + 2HCl + 5{H_2}O\xrightarrow{{}}NaCl + 4B{\left( {OH} \right)_3}$
Properties:
It is a weak monobasic acid acting as Lewis acid that accepts electron from hydroxyl ion. Reaction is as follows:
$B{\left( {OH} \right)_3} + 2HOH\xrightarrow{{}}{\left[ {B{{\left( {OH} \right)}_4}} \right]^ - } + {H_3}{O^ + }$
When boric acid is heated above $370K$, it forms metaboric acid which on further heating yields boric oxide.
${H_3}B{O_3}\xrightarrow{\Delta }HB{O_2}\xrightarrow{\Delta }{B_2}{O_3}$
Boron Hydride; Diborane
Diborane is prepared by the reduction of boron trifluoride with lithium aluminium hydride.
$4B{F_3} + 3LiAl{H_4}\xrightarrow{{}}2{B_2}{H_6} + 3LiF + 3Al{F_3}$
Industrially it is produced by reduction of boron trifluoride with sodium hydride.
$2B{F_3} + 6NaH\xrightarrow{{450K}}{B_2}{H_6} + 6NaF$
Properties:
Diborane is a highly toxic gas that boils at a temperature of $180K$. It catches fire very readily on exposure to air.
${B_2}{H_6} + 3{O_2}\xrightarrow{{}}{B_2}{O_3} + 3{H_2}O$
Diborane is a dimer of boron hydride where four terminal hydrogen atoms and two boron atoms are place in one plane and two bridging hydrogen atoms are out of the plane.
Therefore, there are four terminal $B - H$ bonds (2 centre-2-electrons) and two are brigade bonds of $B - H - B$ having a 3-centre-2-electrons bond.
Aluminium:
It is a bright silvery metal having high tensile strength. It shows typical metallic properties like malleability and ductility.
It has high electrical and thermal conductivity. As for these properties, aluminium is dearly used in everyday life and industries also.
It forms alloys with metals like $Cu,{\text{ }}Mn,{\text{ }}Mg,{\text{ }}Si,{\text{ }}Zn$ etc.
These alloys are used in the manufacture of pipes, tubes, rods, wires, plates, foils, utensils etc.
It is also used for construction purposes, aeroplane parts, transportation industry.
Group -14 Elements
Carbon Family
Group-14 comprises 5 elements namely, carbon, silicon, germanium, tin, lead.
Among all, carbon is the seventeenth most abundant element in the earth’s crust. It exists in free elemental form as coal, graphite and diamond and in combined form as hydrocarbons, carbonates, carbon dioxide gas etc.
Silicon is the second abundant element in earth’s crust. It is present in the form of silica and silicates.
Germanium is found in traces only while tin mainly exists as cassiterite, $Sn{O_2}$ and lead as galena $PbS$.
Electronic Configuration:
The general electronic configuration of elements carbon family is $n{s^2}n{p^2}$
Covalent Radius:
On moving down the group, the size of the element increases but the increase is uneven.
There’s a considerable increase in size from carbon to silicon but a small increase in size is observed from silicon to lead.
This is because of the presence of completely filled d and f-orbitals.
Ionisation Enthalpy:
It is higher for group-14 elements than corresponding group-13 elements. Generally, on moving down the group, the ionisation enthalpy decreases.
But a small decrease is observed from $Si$ to $Ge$ to $Sn$ and then a slight increase is observed from $Sn$ to $Pb$.
This is because of poor shielding effect of interviewing d and f-orbitals and increase in the covalent radii of the elements.
Electronegativity:
As compared to group-13, carbon family members are more electronegative in nature and within the group, carbon is most electronegative while from $Si$ to $Pb$, the electronegativity remains almost the same.
Physical Properties:
Carbon family is a mixture of metals, metalloids and non-metals.
Carbon is non-metals while silicon and germanium are a metalloid and tin and lead are soft metals.
Group-14 elements have melting points and boiling points higher than corresponding group-13 elements.
Chemical Reactivity:
Oxidation State:
Common oxidation states shown by group-14 elements are $ + 2$ and $ + 4$.
Carbon and silicon mostly show $ + 4$ oxidation state. Carbon is also seen to show negative oxidation state.
Germanium forms stable compounds in $+4$ oxidation state only and few compounds in $ + 2$ oxidation state.
Tin forms compounds in both the oxidation states.
Lead compounds are stable in $ + 2$ oxidation state while $ + 4$ oxidation state are strong oxidising agents.
Reactivity Towards Oxygen:
All the elements in reaction with oxygen form two types of oxides- monoxide and dioxide. $MO$ and $M{O_2}$.
Oxides in higher oxidation states of elements are more acidic in nature than the oxides in lower oxidation states.
$C{O_2}{\text{, S}}i{O_2}{\text{, }}Ge{O_2}$ are acidic in nature while $Sn{O_2}{\text{ }}and{\text{ }}Pb{O_2}$ are amphoteric in nature.
Reaction Towards Water:
Carbon, silicon and germanium are unreactive towards water while tin decomposes in steam to form oxide and liberate hydrogen gas.
$Sn + 2{H_2}O\xrightarrow{\Delta }Sn{O_2} + 2{H_2}$
Lead is also unaffected by the action of water due to formation of protective oxide film over metal.
Reaction Towards Halogens:
Dihalide and tetrahalide compounds are formed by group-14 elements. $M{X_2}{\text{ }}and{\text{ }}M{X_4}$ .
Most of the $M{X_4}$ are covalent in nature having central atom with $s{p^3}$ hybridisation and tetrahedral shape.
Exceptions are $Sn{F_4}$ and $Pb{F_4}$ are ionic in nature. $Pb{I_4}$ doesn’t exist in nature as $Pb - I$ bond which is initially formed is so weak that it doesn’t release enough energy to unpair electrons for further bond formation.
As a result, the stability of dihalide increases down the group.
Anomalous Behaviour of Carbon:
Carbon has a small size, high electronegativity, higher ionisation enthalpies and lacks d-orbitals.
As a result, it can have its covalence till four while other elements can expand their covalence.
Carbon can also form $p\pi - p\pi$ multiple bonding. Heavier metals don’t show these bonding because of their large size and they diffuse to have effective overlapping.
Carbon has self-linking property, where carbon atoms link with one another through covalent bonds to form chains and rings. This property is called catenation.
Due to this property of catenation, carbon exists in various allotropic forms.
Allotropes of Carbon:
Graphite and diamond are two well-known allotropes of carbon. The third form of carbon is fullerene which was discovered in the year 1985.
Diamond:
This is the crystalline form of carbon where each carbon atom is $s{p^3}$ hybridised and exists in a tetrahedral shape. The $C - C$ bond length in diamond is 154 pm
In diamond, each carbon is joined to four other carbons which when extended in space form a rigid three-dimensional structure. Diamond is the hardest substance known on earth. It is used for sharpening hard tools, in making dyes and in the manufacturing of tungsten filaments that are used in electric bulbs and tube lights.
Graphite:
This is the layered structure of carbon where layers are held together by vander waal forces.
Each carbon is $s{p^2}$ hybridised and is linked with three carbon atoms via sigma bonds keeping forth electron to form $\pi $ bond.
This electron is delocalised over the whole sheet and is responsible for the electrical conductivity of graphite.
The $C - C$ bond length in graphite is $141.5{\text{ }}pm$ . Graphite is soft and slippery and used as a dry lubricant in heat-resistant machineries.
Fullerenes:
When graphite is heated in an electric arc in the presence of inner gas such as helium or argon, a sooty material is formed by the condensation of vaporised carbon. That sooty material consists of mainly ${C_6}_0$ with a smaller quantity of ${C_{70}}$ and traces of fullerenes.
Fullerene is cage-like molecules and have a shape of soccer ball and is also called as buckminsterfullerene.
Structurally, it is composed of 20 six- membered rings and 12 five-membered ring fused together. The carbon atom is $s{p^2}$ hybridised and is attached to three carbon atoms via sigma bonding.
The fourth electron left is delocalised over the structure and gives aromatic character to the fullerene molecule.
Out of all the allotropes of carbon, graphite is thermodynamically more stable than rest of them.
Some Important Compounds of Carbon:
Carbon Monoxide:
It is a colourless, odourless gas which is water-insoluble. It is prepared by direct oxidation of carbon in a restricted supply of oxygen.
$2C\left( s \right) + {O_2}\left( g \right)\xrightarrow{\Delta }2CO\left( g \right)$
It can also be produced by the dehydration of formic acid in the presence of concentrated sulphuric acid. The $CO$ produced by this reaction is in its pure form.
$HCOOH\xrightarrow[{{H_2}S{O_4}}]{{373K}}{H_2}O + CO$
Commercially, it is prepared by the action of steam over hot Coke. A mixture of $CO$and ${H_2}$ gas is obtained which is called as water gas or synthetic gas.
$C + {H_2}O\xrightarrow{{473 - 1273K}}CO + {H_2}$
This prevents the haemoglobin to transport oxygen to tissues of our body and can cause serious health issues and ultimately death also.
Carbon Dioxide:
This is produced by the complete combustion of carbon.
$C + {O_2}\xrightarrow{\Delta }C{O_2}$
Synthetically, it can be prepared by reaction between calcium carbonate with dilute hydrochloric acid.
$CaC{O_3} + 2HCl\xrightarrow{{}}CaC{l_2} + C{O_2} + {H_2}O$
$C{O_2}$ is a colourless, odourless with low solubility in water. On reaction with water, it forms carbonic acid which is a di basic acid.
$C{O_2}$ is the major reactant for the photosynthesis reaction.
$6C{O_2} + 12{H_2}O\xrightarrow[{chlorophyll}]{{hv}}{C_6}{H_{12}}{O_6} + 6{O_2} + 6{H_2}O$
$C{O_2}$ is non-poisonous to human health but is a major cause for green-house effect and increased global warming.
Solid form of $C{O_2}$ is known as dry ice which is widely used as a refrigerant for ice creams and frozen foods. $C{O_2}$ is also used in fire extinguishers.
Some Important Compounds of Silicon:
Silicones:
The repeating unit of silicone is $\left( {{R_2}SiO} \right)$.
Methyl chloride reacts with silicon In the presence of copper as catalyst and temperature at $573K$, Chlorosilanes are formed. Hydrolysis of dimethyldichlorosilane followed by condensation polymerisation yields the straight chain polymer of silicone.
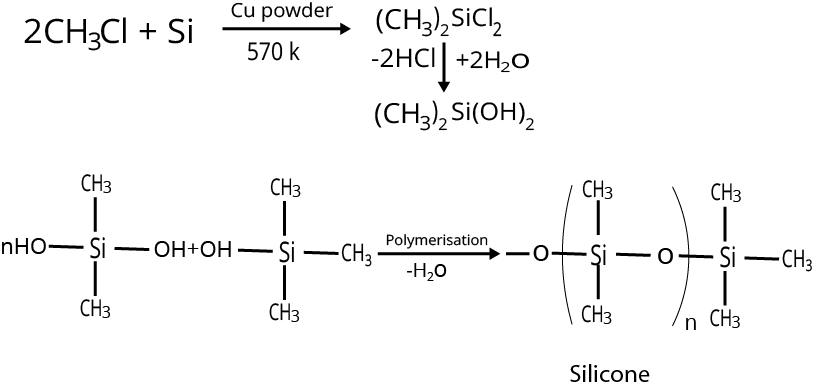
Image: Silicone formation
They have high thermal stability and high dielectric strength and are resistant to oxidation and chemicals.
They are widely used in electric insulators, greases, sealants and for waterproofing of fabrics. They are biocompatible and are used in surgical and cosmetic implants.
Silicates:
The basic structure of silicates is $SiO_4^{4 - }$.
Here, each silicon atom is linked to four oxygen atoms in a tetrahedral manner. When duplicate units are joined together, they form chain, ring, sheet, three-dimensional structure.
The negative charge on silicates is balanced by the positive charge of metal ions.
Silicates are widely used in the manufacture of glass and cement.
Zeolites:
In the structure of silicon dioxide, when few atoms of silicon are replaced by aluminium atoms, the overall structure becomes aluminosilicate and acquires a negative charge.
Cations like $N{a^ + }{\text{, }}{{\text{K}}^{\text{ + }}}{\text{, C}}{a^{2 + }}$ balance this negative charge and a complex is formed. The examples of such complexes are feldspar and zeolites.
Zeolites are extensively used as catalysts on petrochemical industries.
Importance of Chemistry P-Block Elements for NEET
The chapter on P Block Elements has a lot of significance for Class 11 and Class 12 students since it is included in the syllabus for NEET. Students have to build a strong conceptual foundation for the chapter and this is where the Class 11 Chemistry P Block Elements notes can help them out.
P Block Elements can be distinguished into different families such as the Nitrogen Family, the Oxygen Family, the Halogen Family and much more. The chapter delves into all the physical and chemical properties of the elements that belong to these families. All the reactions and principles have been explained in a detailed format for the students to comprehend. Students will learn various terms and the significance that they have in the chapter such as Electronic Configuration, Ionic and Atomic Radii, Ionisation Enthalpy, Electronegativity, Atomic & Physical Properties and so much more.
They will be familiarized with different elements that belong to these P Block Families and how they can be achieved with various chemical reactions and processes. All these reactions have practical use and students can score high marks in the examinations by preparing from these revision notes in the best way.
Revision notes for the chapter contain all the necessary details and information about P Block elements and their significance that students can thoroughly study for a better understanding of the chapter.
Benefits of Vedantu’s Redox Reaction Revision Notes for NEET
Vedantu experts have designed the P Block elements Class 11 notes for NEET to help students prepare for this competitive examination. With the help of the notes, students can gain a lot of benefits, some of which are mentioned below.
Revision notes for P Block elements are a great way to start preparing for the NEET entrance examination. The notes have been designed in a precise and concise manner including all the important details that are in the chapter.
Students now have a convenient and efficient resource where they can find conceptual details about the chapters. Hence, there is no doubt that they will have no trouble downloading the notes.
The chapter for P Block elements contains important questions as well as worksheets in the revision notes that students can use in order to practice for their upcoming examinations.
Download Redox Reactions Revision Notes for NEET Preparation
Don’t miss out on the opportunity to score a high rank in NEET examinations. Download the P Block elements notes for NEET and start your preparation journey towards success. All the subtopics, reactions, principles, etc. have been explained in great detail for better comprehension.
Important Related Links for NEET
FAQs on Revision Notes on The P-Block Elements for NEET 2026
1. Which are the toxic elements in the Periodic Table?
The periodic table consists of a few toxic elements that include the radioactive ingredient named Polonium. Also, Mercury is another element which is omnipresent and a very deadly substance. Lead and Arsenic are other examples of toxic elements.
2. How do you explain the term inert gases?
These gases are also known as noble gases. These gases don’t undergo any particular chemical reaction under different conditions. Some of the examples of noble gases include Argon, Helium, Radon, Organesson, Xenon, etc.
3. What are the non-metals in the periodic table?
The non-metals are located on the extreme right section of the Periodic Table. There are different non-metals such as Arsenic, Sulphur, Mercury, Fluorine, Ammonia, etc.
4. Why doesn’t Fluoride exhibit a positive state of oxidation?
Fluorine is considered to be the most electronegative element in the Periodic Table and it doesn’t have any d-orbitals in the valence shells. Hence, expansion of the octet is not possible so there isn’t a positive state of oxidation.

























