Introduction
In the p Block Elements, the last electron enters in the outermost p-orbital. There are six groups, numbering from 13 to 18 in the Periodic table, which constitute the p-block elements. Their valence shell general electronic configuration is ns2 np1-6 (except for He). The p–block contains metals, metalloids as well as non–metals in the periodic table.
The p-Block Elements is an important topic of the NEET Exam. Physical properties, chemical properties, Anomalous properties, Oxidation states and Some important compounds of the B, Al, C and Si are all significant topics. We will explore everything about the characteristics of group 13 and group 14, in this article. This article provides an example of the types of questions that might be asked about this subject.
Important Topics of the p-Block Elements
Electronic configuration
Physical Properties Trend
Chemical Properties Trend
Anomalous Behaviour
Oxidation States
Occurrence
Some Important Compounds of Boron
Aluminium: uses and reactions
Carbon: Allotropic forms
Carbon: Physical & Chemical Properties
Carbon: uses of some important compounds
Some Important Compounds of Silicon
Electronic Configuration of the Elements of Group 13 and Group 14
Occurrence of the Group 13 and 14 Elements
Aluminium is the most abundant metal and 3rd most abundant element (after oxygen and silicon) by mass in the earth's crust. The most important ore of aluminium (Al) is bauxite. Boron is a fairly rare element but occurs as concentrated deposits of borax, kemite and tourmaline crystal. Gallium (Ga) is twice as abundant as boron, but indium and thallium are much less common. All 3 elements (Ga, In and TI) occur as sulfides in traces in zinc and lead sulfide ores.
Carbon is the 17th and silicon the 2nd most abundant element by mass in the earth's crust. Carbon is found both in native and combined states. ‘Silicon’ occurs very widely as silica and a variety of silicate minerals and clays. Germanium is found only in traces in some silver and zinc ores and in some types of coal. But abundance of tin and lead is comparatively low, but occur as concentrated ores. Tin is mined as cassiterite, SnO2 and lead is found as galena ore, PbS.
Anomalous Behaviour of the First Element of Group 13 and 14
Anomalous Behaviour of Boron (B)
Boron has very small atomic radii, hence greater nuclear attraction on the outermost electrons. It has very high ionization energy. This gives boron distinctly nonmetallic character while the rest are metals.
Boron has maximum covalency of 4 due to nonavailability of d-orbitals while the rest have maximum covalency of 6.
Boron alone exhibits allotropy. And Boron shows +3 oxidation state while others can show + 1 and +3 oxidation states.
Boron halides are monomeric while the halides of the other elements are dimeric. ‘B’ does not show an inert pair effect.
Anomalous Behaviour of Carbon (C)
Carbon also differs from the rest of the elements of its group due to its smaller size, higher electronegativity, higher ionization enthalpy and unavailability of d orbitals. It can accommodate only 4 pairs of electrons around it. This would limit the maximum covalence to 4 whereas other members can expand their covalence due to the presence of d orbitals. Also Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings. This property is called catenation. Because C–C bonds are very strong. On moving down the group, the size increases and the tendency to show catenation decreases.
Oxidation States of the Elements of Group 13 and 14
In group 13 we first encounter elements possessing more than one oxidation state and the expected oxidation states are +3 and + 1. Boron shows +3 oxidation state in all its compounds. Other members show +3 and + 1 oxidation states. The stability of + 1 oxidation state increases from Al to Tl and the stability of +3 oxidation state decreases from Al to Tl. The +3 stability decreases due to increase in inert pair effect.
C and Si show oxidation state of +4 while Ge, Sn, Pb show oxidation states of both +2 and +4 due to the Inert Pair Effect.
Trends in the Variation of Properties of Group 13 and 14 Elements
Trends in the Chemical Reactivity of Group 13 and 14 Elements
Boron and its some important compounds: Borax, Boric Acids, Boron Hydrides
Boron
Boron is obtained these days by the electrolysis of a fused mixture containing boric anhydride, magnesium oxide and magnesium fluoride at 1100°C.
Elemental boron exists in two allotropic forms - grey black, nonmetallic and a dark
brown amorphous powder.
Boron is used as a deoxidiser in the casting of copper and as a catalytic agent. And used for making boron steels which are very hard and are used as control rods in atomic reactors.
Aluminium: Uses, Reactions with Acids and Alkalis
Aluminium wire is used in transmission lines and coils for dynamos and motors as well as for making silvery paints for covering iron and other materials. Al is used in the thermite process for the extraction of Cr, Mn, etc. Due to its lightness, good conductivity and resistance to corrosion, it is used for making alloys which find applications in industries and arts.
The oxidation potential of Aluminium is 1.66 V. Thus, it is strongly electropositive, very reactive and a powerful reducing agent. It dissolves in HCI (dil.and conc.) and dil. sulphuric acid, evolving hydrogen.
2Al + 6HCI → 2AICI3 + 3H2
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Aluminium is attacked by caustic alkalies with the evolution of hydrogen gas.
2AI + 6NaOH → 2Na3AIO3 + 3H2
Carbon: allotropic forms, physical and chemical properties, uses of some important compounds: oxides
Allotropic Forms
The tendency of catenation decreases as C >> Si > Ge~Sn >Pb. Carbon provides the best examples of allotropy. There are three important allotropes of carbon: diamond, graphite and fullerenes.
Physical and Chemical Properties
All the allotropic forms of carbon burn in air or oxygen.
C + O2 → CO2 (when oxygen supply is sufficient)
2C + O2 → 2CO (when oxygen supply is insufficient)
Carbon combines with sulfur when the vapors of sulfur are passed over red hot carbon forming carbon disulphide.
C + 2S → CS2
Uses of Some Important Compounds: Oxides
Important Compounds of Silicon: Silicon Tetrachloride, Silicones, Silicates and Zeolites, their Uses
1. Silicon Tetrachloride
SiCl4 can be prepared by passing dry chlorine over an intimate mixture of silica and carbon by heating to 1675 K in a porcelain tube.
SiO2 + 2Cl2 + 2C → SiCl4 + 2CO
It is used in the production of semiconducting silicon and also used as a starting material in the synthesis of silica gel, a binder of ceramic materials.
2. Silicones
Silicones are synthetic organosilicon compounds containing repeated unit R2SiO held by Si – O – Si linkages. These are water repellent, heat resistant, chemically inert, resistant to oxidation and attack by organic acids and are good electrical insulators.
Silicones are formed by the hydrolysis of alkyl or aryl (- R) substituted chlorosilanes and their subsequent polymerisation.
3. Silicates
Silicates are formed by heating ‘metal oxide or metal carbonates’ with the sand. Basic structural unit of silicates is SiO44–.
There are following types of silicates - orthosilicates, pyrosilicates, cyclic silicates, chain silicates, two dimensional sheet silicates, three dimensional sheet silicates.
4. Zeolites
Zeolites are 3-D Aluminosilicates where Al-atoms replace Si-atoms in Silica structure. It is known as Shape selective catalyst.
ZSM-5(A type of zeolite) widely used as a catalyst in petrochemical industries for cracking of hydrocarbons and isomerisation.
Zeolites are used as ion exchangers in softening of “hard” water:
2NaZ (s) + Ca2+ (aq) ⇌ CaZ2 (s) + 2 Na+ (aq)
Solved Examples from the Chapter
Example 1: Borax is used as a cleansing agent because on dissolving in water, it gives:
(a) Alkaline Solution (b) Acidic Solution
(c) Neutral Solution (d) Bleaching Solution
Solution:
Borax on dissolving in water, it gives an alkaline solution. Thus, the correct answer is option (a) alkaline solution. The reaction can be represented as:
Na2B4O7 + 7H2O ⇌ 2NaOH + 4H3BO3
Here, NaOH is a strong base while H3BO3 is a weak acid.
Key point to remember: The solution of borax is alkaline in nature because of its hydrolysis (salt of strong alkali and weak acid). That is why Borax is used as a cleansing agent.
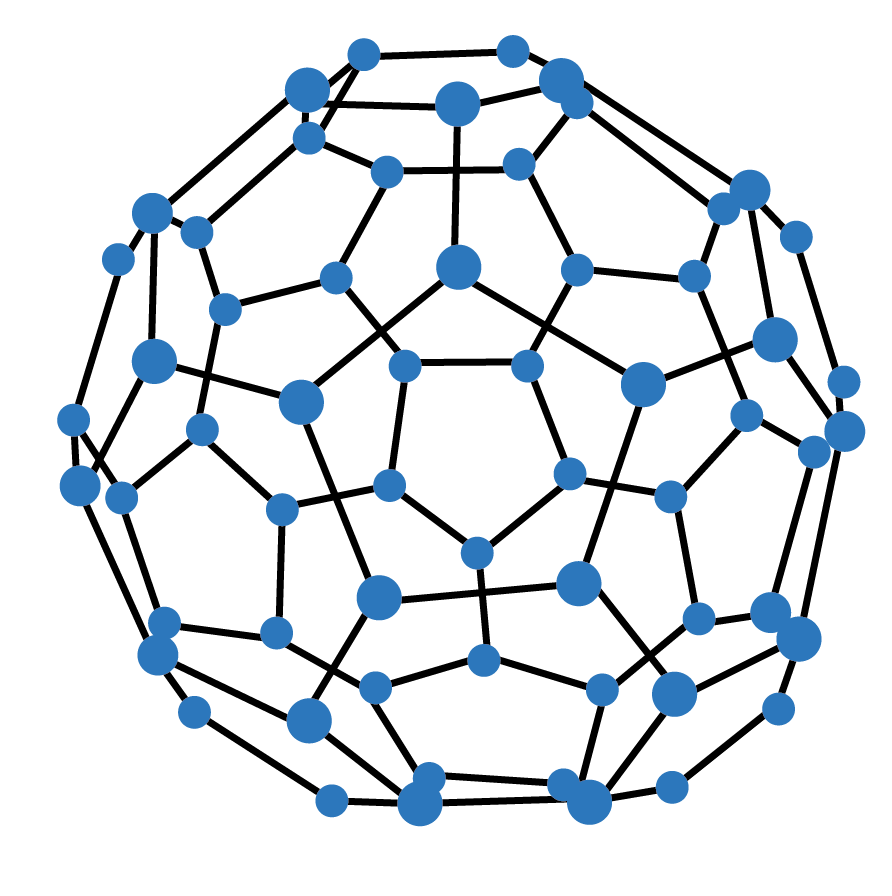
Example 2: Which of the following is Buckminsterfullerene:
(a) Diamond (b) Graphite
(c) C60 (d) Bone Charcoal
Solution: Fullerene is an allotrope of carbon, consisting of spherical C60 molecules with the extraordinary shape of a soccer ball. It is made from interlocking hexagonal and pentagonal rings of carbon atoms with each atom sp2 hybridized and bonded to three other atoms. Thus, the correct answer is option (c) C60.
Key point to remember: Allotropy is defined as the property due to which an element exists in two or more forms which differ in their physical and some of the chemical properties and the various forms are called allotropes. Fullerene is also the allotrope of carbon with carbon molecules C60.
Solved Questions From The Previous Year Question Papers
Question 1: Which of the following are the correct statements?
(a) CO2 (g) is used as a refrigerant for ice-cream and frozen food.
(b) The structure of C60 contains 12 six carbon rings and 20 five carbon rings.
(c) ZSM-5, is a type of zeolite, used to convert alcohols into gasoline.
(d) CO is colorless and odourless gas.
A. (b) and (c) only B. (c) and (d) only
C. (a), (b) and (c) only D. (a) and (c) only
Solution: CO2 (s) is dry ice not CO2 (g), so it is not used as a refrigerant for ice-cream and frozen food.
The structure of C60 contains 20 six carbon rings and 12 five carbon rings.
ZSM-5 is a zeolite which is used to convert alcohol directly into gasoline (petrol).
Carbon monoxide CO (g) is a colorless and odorless gas.
Hence, the correct answer is option B. (c) and (d) only.
Trick: Generally oxides of metals are basic in nature. When we move from top to bottom in a group, the basic character of oxides increases.
Question 2: In which of the following, the incorrect statement is?
(a) SnF4 is ionic in nature
(b) PbF4 is covalent in nature
(c) SiCl4 is easily hydrolysed
(d) GeX4 (X = F, Cl, Br, and I) is more stable than GeX2
Solution:
(a) Sn is a very large atom and F is very small in size so according to Fajan’s rule, SnF4 is ionic in nature. Hence, it is a correct statement.
(b) Pb is a very large atom and F is very small in size so according to Fajan’s rule, PbF4 is ionic in nature and not covalent in nature. Hence, it is an incorrect statement.
(c) SiCl4 is not a very stable compound and we know that compounds which are unstable in nature can be easily dissociated or hydrolysed. Hence, it is a correct statement.
(d) GeX4 (X = F, Cl, Br, and I) is more stable than GeX2 because in case of GeX4 all the orbitals are fully filled. Hence, it is a correct statement.
Trick: SnF4 and PbF4 both are ionic in nature according to Fajan’s rule.
Question 3: In which of the following, the element unable to form MF63– ion is?
(a) Ga (b) B (c) In (d) Al
Solution: All the given elements B, Al, Ga and In belong to the same group i.e. group 13 in the periodic table. ‘B’ belongs to the 2nd period where only s and p orbital are available. s orbital can have a maximum of 2 electrons and p orbital can have a maximum of 6 electrons. Therefore, ‘B' has no vacant d -orbitals in its valence shell that is why it cannot extend its covalency beyond 4. i.e. 'B' cannot form an ion like MF63– i.e. BF63–. Hence, the correct answer is option (b) B.
Trick: The smaller the size of an atom or element, the more difficult it would be to form bonds with 6 atoms due to steric hindrance. The one which is smallest in size among these, will not be able to form the complex. The non availability of d orbital makes the element unfit for formation of MF63– complexes.
Practice Questions
Question 1: Which of the following compounds is formed in borax bead test?
(a) Metaborate (b) Tetraborate .
(c) Double oxide (d) Orthoborate
Answer: (a) Metaborate
Question 2: Ge (II) compounds are the powerful reducing agents whereas Pb (IV) compounds are strong oxidants. The reason is:
(a) Pb is more electronegative than that of Ge
(b) Ionization potential of Pb is less than that of Ge
(c) Ionic radii of Pb2+ and Pb4+ are larger than those of Ge2+ and Ge4+
(d) More pronounced inert pair effect in Pb than in Ge.
Answer: (d) More pronounced inert pair effect in Pb than in Ge.
Conclusion
In this article, we studied the Group 13 elements (Boron Family) and Group 14 elements (Carbon Family) and why they are called p-block elements. And we studied their electronic configuration, occurrence, oxidation states and the abnormal behaviour of the first element of each group. And also we know the physical and chemical properties of the group 13 and group 14 elements as well as their some common compounds.
Hence, this chapter is important not only for competitive exams like JEE or NEET but also in better understanding the study of p -block elements and their compounds.
NEET Chapter: The p Block Elements

 Share
ShareFAQs on NEET Chapter: The p Block Elements
1. Why do elements of group 14 not form ions?
Elements of Group 14 have 4 electrons in their outermost shell. So, they can either gain or lose 4 electrons to complete their octet. But, they require a large amount of energy to lose or gain 4 electrons. Therefore, they do not form ions, instead form covalent bonds by sharing electrons with another atom.
2. Why are the group 13 elements part of the p -Block elements?
The elements belong to group 13 also known as Boron Family and they have 3 electrons in their outermost shell. So, the last electron enters in the outermost p orbital that is why elements of group 13 are the part of p -Block elements. General electronic configuration of the p -Block element is ns2np1.
3. What is the oxidation state of group 13 elements?
The elements of group 13 are B, Al, Ga, In, Tl. The oxidation states of group 13 elements are +3 and +1. The stability of + 3 oxidation state decreases from Al to Tl while stability of oxidation state increases from Al to Tl.




















 Watch Video
Watch Video