
You are given benzene, conc. ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$ and ${\rm{NaOH}}$. Write the equations for the preparation of phenol using these reagents.
Answer
233.1k+ views
Hint: In synthesis two or more compounds or elements combine to make a more complex substance. To form benzene sulphonic acid we have to use benzene and conc. sulphuric acid in presence of heat.
Complete step by step answer:
We know that benzene is an organic compound and molecular formula is ${{\rm{C}}_6}{{\rm{H}}_{\rm{6}}}$. Benzene reacts with concentrated sulphuric acid $\left({{\rm{conc}}{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}} \right)$ in presence of heat and form benzene sulphonic acid. Benzene sulphonic acid is an organosulfur compound with formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}{{\rm{O}}_{\rm{3}}}{\rm{S}}$. Now, we write the reaction between benzene and conc. sulphuric acid in presence of heat is as follows:
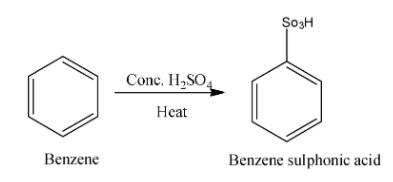
After formation of benzene sulphonic acid it is then heated with sodium hydroxide and form sodium phenoxide. Now we write the reaction between benzene sulphonic acid and sodium hydroxide in presence of heat is as follows:

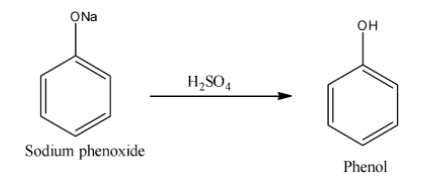
After formation of sodium phenoxide hydrolysis of sulphuric acid given phenol. Sodium phenoxide is an organic compound with molecular formula ${\rm{NaO}}{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}$. For the formation of phenol from sodium phenoxide heat is not required. We write the reaction of formation of phenol is as follows:
Additional Information: Phenol is a volatile solid that is found in white crystalline form. Phenol contains one $ - {\rm{OH}}$ group. Phenol is an aromatic compound and molecular formula is ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{\rm{6}}}{\rm{OH}}$. Phenols are used as disinfectants, mouthwash, household cleaners, antiseptics etc.
Note: Hydrolysis is a chemical reaction which one or more chemical bonds break up by a molecule of water. The term is commonly used for replacement, reduction and solving reactions in which nucleophile is water. In industry hydrolysis is commonly used to break down chemicals into smaller fractions or parts. Do not confuse this formation of phenol reaction with sulfonation reaction.
Complete step by step answer:
We know that benzene is an organic compound and molecular formula is ${{\rm{C}}_6}{{\rm{H}}_{\rm{6}}}$. Benzene reacts with concentrated sulphuric acid $\left({{\rm{conc}}{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}} \right)$ in presence of heat and form benzene sulphonic acid. Benzene sulphonic acid is an organosulfur compound with formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}{{\rm{O}}_{\rm{3}}}{\rm{S}}$. Now, we write the reaction between benzene and conc. sulphuric acid in presence of heat is as follows:

After formation of benzene sulphonic acid it is then heated with sodium hydroxide and form sodium phenoxide. Now we write the reaction between benzene sulphonic acid and sodium hydroxide in presence of heat is as follows:

After formation of sodium phenoxide hydrolysis of sulphuric acid given phenol. Sodium phenoxide is an organic compound with molecular formula ${\rm{NaO}}{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}$. For the formation of phenol from sodium phenoxide heat is not required. We write the reaction of formation of phenol is as follows:

Additional Information: Phenol is a volatile solid that is found in white crystalline form. Phenol contains one $ - {\rm{OH}}$ group. Phenol is an aromatic compound and molecular formula is ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{\rm{6}}}{\rm{OH}}$. Phenols are used as disinfectants, mouthwash, household cleaners, antiseptics etc.
Note: Hydrolysis is a chemical reaction which one or more chemical bonds break up by a molecule of water. The term is commonly used for replacement, reduction and solving reactions in which nucleophile is water. In industry hydrolysis is commonly used to break down chemicals into smaller fractions or parts. Do not confuse this formation of phenol reaction with sulfonation reaction.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




