
Which one of the following is the correct formula of dichlorodiphenyltrichloroethane?
A. 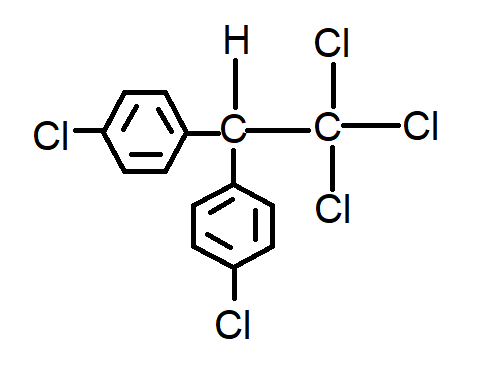
B. 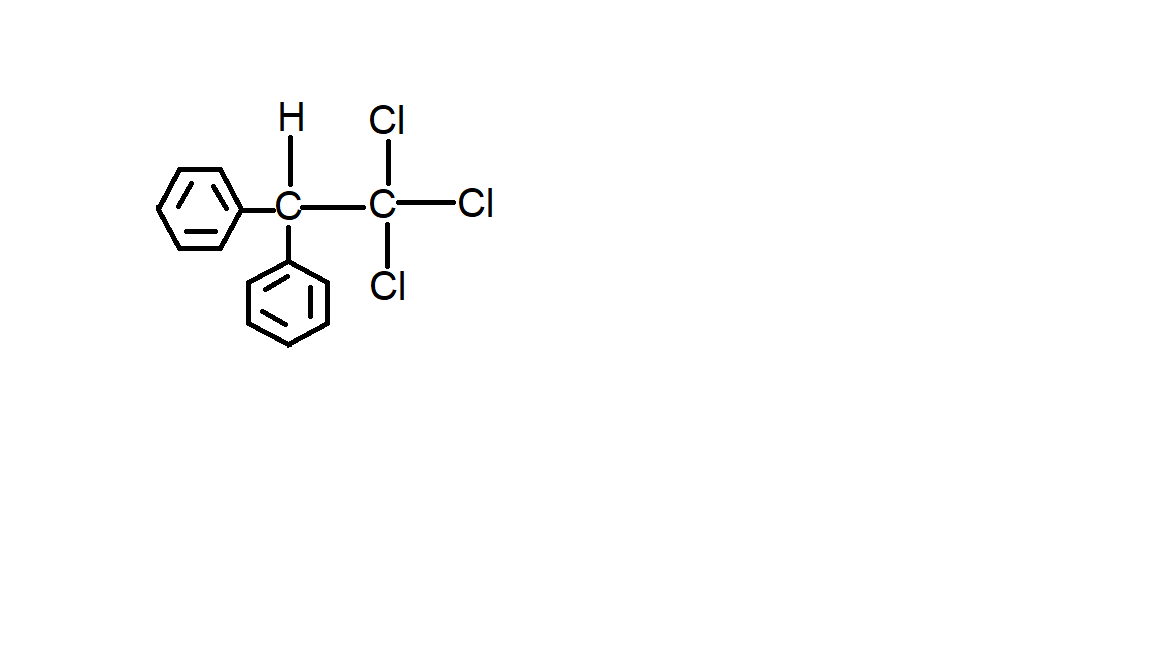
C. 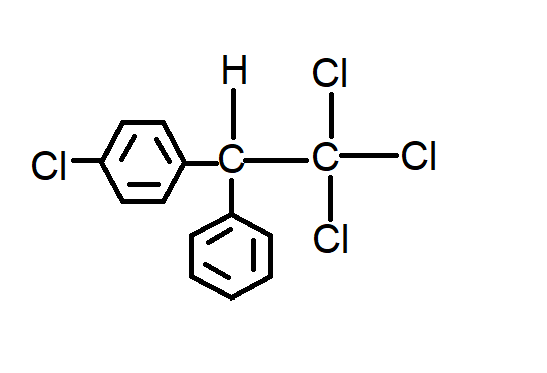
D. 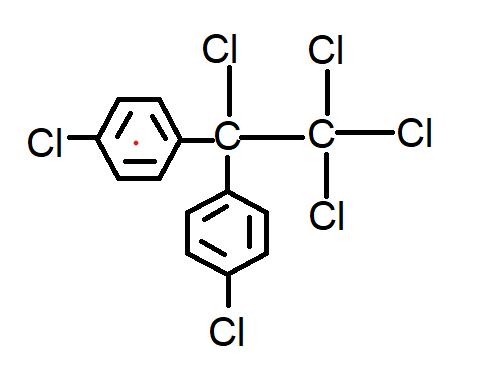
Answer
233.1k+ views
Hint: Naming of organic compounds in organic chemistry is based on the IUPAC nomenclature. The IUPAC nomenclature follows some set of rules to give the name of the compound. Some compounds are known by the IUPAC name whereas some compounds are popular by their common names. The IUPAC name help in depicting the arrangement of atoms in a compound.
Complete step-by-step answer:The given name in the question is based on IUPAC nomenclature. Hence apply the rules of IUPAC nomenclature and identify the correct structure.
The naming is divided into three parts
-Naming of parent chain which is the chain having maximum number of carbon atoms.
-Naming of functional groups if present any
-Naming of side substituents along with correct identification of position number of these substituents.
In dichlorodiphenyltrichloroethane, ethane represents the parent chain having \[2\] carbon atoms. Thus, named as 'eth'
Dichlorodiphenyl trichloro is the name given to the side substituents. There are two side substituents present
One is chloro which are \[3\] in number hence named trichloro
Other is chlorobenzene. In IUPAC nomenclature, benzene as a substituent is named as phenyl. Dichlorodiphenyl refers to two benzene rings with chlorine. The benzene ring is attached to ethane carbon through its para position.
Option B is incorrect because the chlorine atom is not attached to the benzene ring.
Option C has chlorine on only one benzene ring but it should be on both.
Option D has chlorine on both the rings but there are \[4\] chlorine attached to the parent chain which is incorrect.
Option ‘A’ is correct
Note: ‘ane’ represents alkane where the carbon atoms are connected through a single bond. Ene represents double bond and yne represents triple bond. Meth means one carbon atom in the parent chain. Eth means two, prop means three, but means four and pent means five.
Complete step-by-step answer:The given name in the question is based on IUPAC nomenclature. Hence apply the rules of IUPAC nomenclature and identify the correct structure.
The naming is divided into three parts
-Naming of parent chain which is the chain having maximum number of carbon atoms.
-Naming of functional groups if present any
-Naming of side substituents along with correct identification of position number of these substituents.
In dichlorodiphenyltrichloroethane, ethane represents the parent chain having \[2\] carbon atoms. Thus, named as 'eth'
Dichlorodiphenyl trichloro is the name given to the side substituents. There are two side substituents present
One is chloro which are \[3\] in number hence named trichloro
Other is chlorobenzene. In IUPAC nomenclature, benzene as a substituent is named as phenyl. Dichlorodiphenyl refers to two benzene rings with chlorine. The benzene ring is attached to ethane carbon through its para position.
Option B is incorrect because the chlorine atom is not attached to the benzene ring.
Option C has chlorine on only one benzene ring but it should be on both.
Option D has chlorine on both the rings but there are \[4\] chlorine attached to the parent chain which is incorrect.
Option ‘A’ is correct
Note: ‘ane’ represents alkane where the carbon atoms are connected through a single bond. Ene represents double bond and yne represents triple bond. Meth means one carbon atom in the parent chain. Eth means two, prop means three, but means four and pent means five.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




